
Eckhard U Alt
Technical University of Munich, Germany Tulane University, USA
Title: Can we revert the loss of cardiomyocytes in heart failure?
Biography
Biography: Eckhard U Alt
Abstract
Despite revascularization and improved drug therapy, some patients with post myocardial infarction experience a critical loss of functional myocardium leading to remodeling and progression towards heart failure. As the heart is a terminally differentiated organ, renewal and replacement of dead myocardial cells occurs through the intrinsic regenerative potential situated in every organ. As recent research has revealed, the regenerative power of pluripotent stem cells is located throughout the body in small vessels, these cells are able to differentiate into all of the three germ layers. In addition, in every organ we find so-called progenitor cells, which in the heart also constitute the reserve army to replace dying myocardial cells. In light of heart failure with increased ischemia and the survival rate of myocardial cells is significantly reduced, representing an increased turnover and challenge to the stem cells located within the heart. As the ability for stem cells to divide is limited to about 50 to 70 divisions by the telomeres, the regenerative potential in a heart can be exhausted and the functional consequence is a replacement of parenchyma (cardiomyocyte) by fibrous tissue (mesenchyme). Up to now the global understanding why and how cardio myocytes can be replaced is controversial and by most physicians, scientists, and patients obscured. However, the principal of universal stem cells within the body allows obtaining stem cells from one organ, which is not critical such as adipose tissue, to isolate those stem cells and to apply them to the organ in need. The local microenvironment guides the new stem cells through a differentiation program and enables cells to obtain the properties of new cardiomyocytes that completely integrate and synchronize with existing myocardium. The mechanisms of differentiation follow exactly the embryonic differentiation pass way. Hence, it is possible to renew cardiomyocytes by autologous, early stem cells such as the ones that can be retrieved from the stroma of adipose tissue. The mechanism and epigenetic steps of differentiation including results on myocardial regeneration will be presented.
Image:
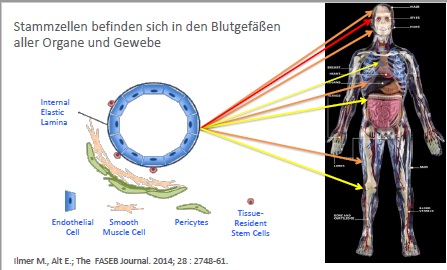
Stem cells reside in virtually all post-natal organs and tissues.
References :
1. Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. & Alt, E. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European heart journal, 28(21), 2667-2677.
2. Sadat, S., Gehmert, S., Song, Y. H., Yen, Y., Bai, X., Gaiser, S., ... & Alt, E. (2007). The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical and biophysical research communications, 363(3), 674-679.
3. Bai, X., Yan, Y., Song, Y. H., Seidensticker, M., Rabinovich, B., Metzele, R., ... & Alt, E. (2009). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European heart journal, ehp568.
4. Song, Y. H., Gehmert, S., Sadat, S., Pinkernell, K., Bai, X., Matthias, N., & Alt, E. (2007). VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochemical and biophysical research communications, 354(4), 999-1003.
5. Alt, E., Yan, Y., Gehmert, S., Song, Y. H., Altman, A., Gehmert, S., ... & Bai, X. (2011). Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colonyâ€forming potential. Biology of the Cell, 103(4), 197-208.
6. Bai, X., & Alt, E. (2010). Myocardial regeneration potential of adipose tissue-derived stem cells. Biochemical and biophysical research communications, 401(3), 321-326.
7. Bai, X., Ma, J., Pan, Z., Song, Y. H., Freyberg, S., Yan, Y., ... & Alt, E. (2007). Electrophysiological properties of human adipose tissue-derived stem cells. American Journal of Physiology-Cell Physiology, 293(5), C1539-C1550.
8. Bai, X., Yan, Y., Coleman, M., Wu, G., Rabinovich, B., Seidensticker, M., & Alt, E. (2011). Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Molecular Imaging and Biology, 13(4), 633-645.
9. Ilmer, M., Vykoukal, J., Boiles, A. R., Coleman, M., & Alt, E. (2014). Two sides of the same coin: stem cells in cancer and regenerative medicine. The FASEB Journal, 28(7), 2748-2761.
10. Alt, E., Pinkernell, K., Scharlau, M., Coleman, M., Fotuhi, P., Nabzdyk, C., ... & Song, Y. H. (2010). Effect of freshly isolated autologous tissue resident stromal cells on cardiac function and perfusion following acute myocardial infarction. International journal of cardiology, 144(1), 26-35.
11. Alt, E. U., Senst, C., Murthy, S. N., Slakey, D. P., Dupin, C. L., Chaffin, A. E., ... & Izadpanah, R. (2012). Aging alters tissue resident mesenchymal stem cell properties. Stem cell research, 8(2), 215-225.

