Day 1 :
Keynote Forum
Eckhard U Alt
Technical University of Munich, Germany Tulane University, USA
Keynote: Can we revert the loss of cardiomyocytes in heart failure?
Time : 09:10-09:50

Biography:
Abstract:
Despite revascularization and improved drug therapy, some patients with post myocardial infarction experience a critical loss of functional myocardium leading to remodeling and progression towards heart failure. As the heart is a terminally differentiated organ, renewal and replacement of dead myocardial cells occurs through the intrinsic regenerative potential situated in every organ. As recent research has revealed, the regenerative power of pluripotent stem cells is located throughout the body in small vessels, these cells are able to differentiate into all of the three germ layers. In addition, in every organ we find so-called progenitor cells, which in the heart also constitute the reserve army to replace dying myocardial cells. In light of heart failure with increased ischemia and the survival rate of myocardial cells is significantly reduced, representing an increased turnover and challenge to the stem cells located within the heart. As the ability for stem cells to divide is limited to about 50 to 70 divisions by the telomeres, the regenerative potential in a heart can be exhausted and the functional consequence is a replacement of parenchyma (cardiomyocyte) by fibrous tissue (mesenchyme). Up to now the global understanding why and how cardio myocytes can be replaced is controversial and by most physicians, scientists, and patients obscured. However, the principal of universal stem cells within the body allows obtaining stem cells from one organ, which is not critical such as adipose tissue, to isolate those stem cells and to apply them to the organ in need. The local microenvironment guides the new stem cells through a differentiation program and enables cells to obtain the properties of new cardiomyocytes that completely integrate and synchronize with existing myocardium. The mechanisms of differentiation follow exactly the embryonic differentiation pass way. Hence, it is possible to renew cardiomyocytes by autologous, early stem cells such as the ones that can be retrieved from the stroma of adipose tissue. The mechanism and epigenetic steps of differentiation including results on myocardial regeneration will be presented.
Image:
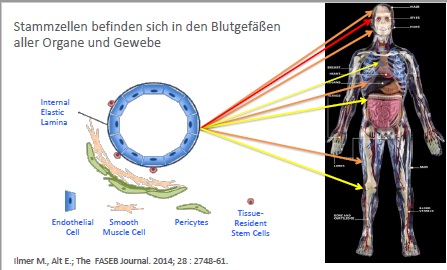
Stem cells reside in virtually all post-natal organs and tissues.
References :
1. Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. & Alt, E. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European heart journal, 28(21), 2667-2677.
2. Sadat, S., Gehmert, S., Song, Y. H., Yen, Y., Bai, X., Gaiser, S., ... & Alt, E. (2007). The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochemical and biophysical research communications, 363(3), 674-679.
3. Bai, X., Yan, Y., Song, Y. H., Seidensticker, M., Rabinovich, B., Metzele, R., ... & Alt, E. (2009). Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. European heart journal, ehp568.
4. Song, Y. H., Gehmert, S., Sadat, S., Pinkernell, K., Bai, X., Matthias, N., & Alt, E. (2007). VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochemical and biophysical research communications, 354(4), 999-1003.
5. Alt, E., Yan, Y., Gehmert, S., Song, Y. H., Altman, A., Gehmert, S., ... & Bai, X. (2011). Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colonyâ€forming potential. Biology of the Cell, 103(4), 197-208.
6. Bai, X., & Alt, E. (2010). Myocardial regeneration potential of adipose tissue-derived stem cells. Biochemical and biophysical research communications, 401(3), 321-326.
7. Bai, X., Ma, J., Pan, Z., Song, Y. H., Freyberg, S., Yan, Y., ... & Alt, E. (2007). Electrophysiological properties of human adipose tissue-derived stem cells. American Journal of Physiology-Cell Physiology, 293(5), C1539-C1550.
8. Bai, X., Yan, Y., Coleman, M., Wu, G., Rabinovich, B., Seidensticker, M., & Alt, E. (2011). Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Molecular Imaging and Biology, 13(4), 633-645.
9. Ilmer, M., Vykoukal, J., Boiles, A. R., Coleman, M., & Alt, E. (2014). Two sides of the same coin: stem cells in cancer and regenerative medicine. The FASEB Journal, 28(7), 2748-2761.
10. Alt, E., Pinkernell, K., Scharlau, M., Coleman, M., Fotuhi, P., Nabzdyk, C., ... & Song, Y. H. (2010). Effect of freshly isolated autologous tissue resident stromal cells on cardiac function and perfusion following acute myocardial infarction. International journal of cardiology, 144(1), 26-35.
11. Alt, E. U., Senst, C., Murthy, S. N., Slakey, D. P., Dupin, C. L., Chaffin, A. E., ... & Izadpanah, R. (2012). Aging alters tissue resident mesenchymal stem cell properties. Stem cell research, 8(2), 215-225.
Keynote Forum
Aris Lacis
University Children Hospital, Latvia
Keynote: Possible therapeutic use of stem cells in wide spectrum of pathologies
Time : 09:50-10:30

Biography:
Aris Lacis is a Cardiac Surgeon and Professor. He completed his MD, PhD and Graduation at Riga Medical Institute. He was a General and Thoracic Surgeon at P. Stradina University Hospital in Riga (1964-1969); Thoracic and Cardiac Surgeon at Latvian Centre for Cardiovascular Surgery (1969-1994). From 1994-2012, he was the Head of Pediatric Cardiology and Cardiac Surgery Clinic at University Children’s Hospital, Riga and; since 2012, he has been a Consulting Professor of this Clinic. He is a Vice President of Latvian Society for Cardiovascular Surgery and; President of Latvian Association for Pediatric Cardiologists. He is an Author of 395 scientific publications, three monographs and 13 patents. He is an Investigator in more than 10 clinical trials including cardio surgical procedures performed under deep hypothermia and hybrid procedures etc.
Abstract:
Context: The promising field of regenerative medicine is working to restore structure and function of damaged tissues and organs. The adult heart represents an attractive candidate for cell-based technologies. While there is a wealth of preclinical and clinical data showing the safety, feasibility, and efficacy of stem cells in adults with acute myocardial infarction and heart failure, less is known about possible implementation of stem cell therapy in infants and children with heart failure due to dilated cardiomyopathy and pulmonary arterial hypertension. The challenges facing cardiac stem cell therapy are multiple. There are uncertainties around the destiny of stem cells after their injection into the blood stream. In particular, it regards migration and homing of implanted cells in the target tissues. As yet unclear is the possible role of sympathetic nervous system in the context of osteoreflexotherapy. There is still no definitive answer to the question on which is the preferred type of stem cells to be used for transplantation in different settings. Since 2008, when we first used autologous bone marrow-derived mononuclear cells (BM-MNCs) in patient with acute myocardial infarction, we have investigated the use of stem cells not only for myocardial regeneration in adults and pediatric patients, but also in adult patients with diabetes mellitus and osteoarthritis.
Aim: Aim of this study is to determine the role of BM-MNCs in management of wide spectrum of pathologies, including critically ill pediatric patients, adult patients with acute myocardial infarction & heart failure and adult patients with osteoarthritis.
Design, Settings & Participants: Two patients (9 and 15 years old) with trisomy 21 and severe pulmonary arterial hypertension due to uncorrected large ventricular septal defects received intrapulmonary BM-MNCs implantation. Radionuclide scintigraphy showed improvement of lungs vascularization during 36 months follow-up. Seven patients (four months–17 years) with dilated idiopathic cardiomyopathy received intra myocardial BM-MNCs injections. During follow-up (up to seven year), we observed improvement of left ventricular ejection fraction (LVEF), decrease of left ventricular end diastolic dimension by echocardiography and cardio-thoracic index at chest X-ray exams, reduction of serum brain-natriuretic peptide serum levels and decrease of the stage of heart failure from stage IV to stage I, by NYHA classification. No peri-procedural harmful side effects were observed. We performed BM-MNCs intracoronary infusion in 101 adult patients with acute myocardial infarction with reduced LVEF and in 14 patients with chronic heart failure. Our results showed statistically significant improvement in LVEF at 12 months. We also infused BM-MNCs to the pancreas directly via branches of splenic artery or superior pancreaticoduodenal artery; we have performed single intra-articular BM-MNCs injections in 70 patients with knee or hip joint osteoarthritis (stage II–III). No adverse effects after the BM-MNC injection were observed. Preliminary analysis showed decrease in pain and other symptoms and statistically significant improvement by clinical scoring system using different questionnaires.
Conclusions: The results are promising and we suggest that BM-MNCs might be used for the stabilization of the adult and pediatric patients to improve symptoms and outcomes or serve as a bridge for heart or lung transplantation or delay joint replacement surgery. It also could be recommended in cases if other more traditional treatment options fail or contraindicated.
Keynote Forum
Wei Hua
Fuwai Hospital & Cardiovascular Institute, China
Keynote: The clinical outcome of cardiac resynchronization therapy in dilated-phase hypertrophic cardiomyopathy
Time : 10:50-11:30

Biography:
Wei Hua is a Professor of Cardiology, Deputy Director at Cardiac Arrhythmia Center, Fuwai Hospital & Cardiovascular Institute, Chinese Academy of Medical Sciences, Peking Union Medical College, China. He completed his MD at Shanghai Medical University in 1985 and then PhD at Graduate School of Peking Union Medical College. He joined Fuwai Hospital & Cardiovascular Institute in 1985 and became full Professor of Cardiology in 1999. He was trained in Cardiac Pacing and Electrophysiology in Royal Melbourne Hospital, Australia, from 1994-1996. His main work is on “Clinical cardiac pacing and electrophysiology, cardiac arrhythmias service”. He is now Vice Chairman of Chinese Society of Pacing and Electrophysiology (CSPE) and Chairman of Cardiac Pacing Committee of CSPE. He is a Fellow of Heart Rhythm Society (FHRS), European Heart Rhythm Association (EHRA) and New York Academy of Sciences.
Abstract:
Statement of the Problem: Clinical trials have demonstrated that cardiac resynchronization therapy (CRT) is effective in patients with non-ischemic cardiomyopathy. However, patients with dilated-phase hypertrophic cardiomyopathy (DHCM) have been generally excluded from such trials. We aimed to compare the clinical outcome of CRT in patients with DHCM, idiopathic dilated cardiomyopathy (IDCM) or ischemic cardiomyopathy (ICM).
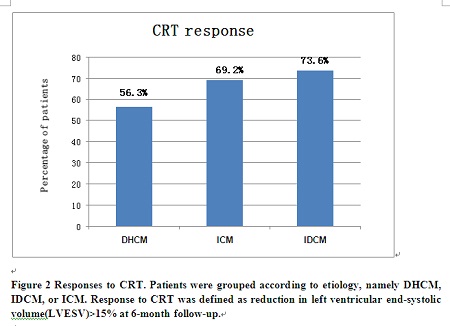
Methodology & Theoretical Orientation: A total of 312 consecutive patients (DHCM=16; IDCM=231; ICM=65) undergoing CRT in Fuwai hospital were studied respectively. Response to CRT was defined as reduction in left ventricular end-systolic volume (LVESV) ≥15% at six-month follow-up.
Findings: Compared with DHCM, IDCM was associated with a lower total mortality [hazards ratio, HR: 0.35 (95% confidence interval, CI 0.13-0.90)], cardiac mortality [HR: 0.29 (95% CI 0.11-0.77)] and total mortality or heart failure (HF) hospitalizations [HR: 0.34 (95% CI 0.17-0.69)], independent of known confounders. Compared with DHCM, the total mortality, cardiac mortality and total mortality or HF hospitalizations favored ICM but were not statistically significant. [HR: 0.59 (95% CI 0.22-1.61); HR: 0.59 (95% CI 0.21-1.63); HR: 0.54 (95% CI 0.26–1.15) respectively]. Response rate to CRT was lower in the DHCM group than the other two groups although the differences didn't reach statistical significance.
Conclusion & Significance: Compared with IDCM, DHCM was associated with a worse outcome after CRT; the clinical outcome of DHCM patients receiving CRT was similar to or even worse than that of ICM patients. These indicate that DHCM behaves very differently after CRT.
Image:
REFERENCES:
1. Hua W, Zhang LF, Wu YF, et al.( 2009) Incidence of sudden cardiac death in China: analysis of 4 regional populations. J Am Coll Cardiol. Sep 15;54(12):1110-8.
2. Yi-Zhou Xu, Yong-Mei Cha, Dali Feng, Wei HUA, et al.( 2012) Impact of Myocardial Scarring on Outcomes of Cardiac Resynchronization Therapy: Extent or Location? J Nucl Med 2012; 53:47-54
3. Hua wei. (2010)Cardiac resynchronization therapy for chronic heart failure in China:guideline and practice(Editorial).Chinese Medical Journal ,123(17): 2293-2294.
4. HUA Wei, HONGXIA Niu, XIAOHAN Fan, et al. (2012) Preventive Effectiveness of Implantable Cardioverter Defibrillator in Reducing Sudden Cardiac Death in the Chinese Population: A Multicenter Trial of ICD Therapy versus Non-ICD Therapy. JCE, 2012, 23(S1): S5-S9
5. HUA Wei, WANG Dong-mei, CAI Lin, et al. (2012) A prospective study to evaluate the efficacy of an intracardiac electrogram-based atrioventricular and interventricular intervals optimization method in cardiac resynchronization therapy. Chinese Medical Journal. 2012, 125(3): 428-433
- Heart Diseases and Heart Failure | Cardiovascular Medicine| Cardiac Surgery and Angiography| Cardiac Arrythmias and Clinical Electrophysiology | Case Study on Cardiology
Location: London, UK

Chair
Lingfang Zeng
Kings College London, UK

Co-Chair
Sherif A S A Mansour
Ain Shams University, Egypt
Session Introduction
Sunny Po
University of Oklahoma Health Sciences Center, USA
Title: Autonomic neuromodulaiton to treat atrial fibrillation
Time : 11:30-12:00

Biography:
Sunny Po is a Cardiac Electro-physiologist and has his expertise in “The effects of the autonomic nervous system on cardiac arrhythmias”. He has discovered that simultaneous activation of the sympathetic and parasympathetic nervous system plays a great role in triggering atrial fibrillation. He also pioneered the therapy of using low-level vagal stimulation at the strength not slowing the sinus rate or atrioventricular conduction to treat atrial fibrillation.
Abstract:
Atrial fibrillation (AF) is the most commonly encountered arrhythmia. Catheter or surgical ablation, aiming at isolating the pulmonary veins to eliminate the triggers and substrate of AF is the therapy of choice for drug-refractory AF. Despite the advances of the ablation technologies, the success rate of AF ablation remains disappointing. It is evident that pulmonary vein isolation (PVI) is not enough to treat even the earliest stage of AF, paroxysmal AF, not to speak more advanced stages of AF. It is known that simultaneous activation of the sympathetic and parasympathetic nervous system plays a major role in the initiation and maintenance of AF. Interventions targeting these neural elements have been shown to favorably affect the clinical course of AF. Multiple preclinical studies demonstrated that low-level vagal stimulation at the strength not slowing the sinus or atrio-ventricular conduction was capable of suppressing AF initiation as well as terminating AF. Injection of botulinum toxin into major atrial ganglionated plexi, the integration centers of the cardiac autonomic nervous system, not only prevented post-operative AF in paroxysmal AF patients undergoing open-heart surgeries but also significantly suppressed the progression of AF in these patients. Recently, a novel therapy by transcutaneous stimulation of the auricular branch of the vagus nerve demonstrated its efficacy on shortening the AF duration, prolonging the atrial effective refractory period as well as suppressing the inflammatory markers such as TNF-α and CRP in paroxysmal AF patients undergoing AF ablation. Results of these recent clinical studies indicate that autonomic neural modulation may be a novel and noninvasive therapy for patients suffering from drug-refractory AF.
References :
1. Qin M, Liu X, Jiang WF, Wu SH, Zhang XD, Po SS. Vagal response during pulmonary vein isolation: Re-recognized its characteristics and implications in lone paroxysmal atrial fibrillation. Int J Cardiol. 2016 May 15;211:7-13.
2. Lo LW, Chang HY, Scherlag BJ, Lin YJ, Chou YH, Lin WL, Chen SA, Po SS. Temporary Suppression of Cardiac Ganglionated Plexi Leads to Long-Term Suppression of Atrial Fibrillation: Evidence of Early Autonomic Intervention to Break the Vicious Cycle of "AF Begets AF". J Am Heart Assoc. 2016 Jul 5;5(7).
3. Zhang L, Po SS, Wang H, Scherlag BJ, Li H, Sun J, Lu Y, Ma Y, Hou Y.Autonomic Remodeling: How Atrial Fibrillation Begets Atrial Fibrillation in the First 24 Hours. J Cardiovasc Pharmacol. 2015 Sep;66(3):307-15.
4. Yu L, Dyer JW, Scherlag BJ, Stavrakis S, Sha Y, Sheng X, Garabelli P, Jacobson J, Po SS. The use of low-level electromagnetic fields to suppress atrial fibrillation. Heart Rhythm. 2015 Apr;12(4):809-17.
5. Gao M, Zhang L, Scherlag BJ, Huang B, Stavrakis S, Hou YM, Hou Y,Po SS. Low-level vagosympathetic trunk stimulation inhibits atrial fibrillation in a rabbit model of obstructive sleep apnea. Heart Rhythm. 2015 Apr;12(4):818-24.
6. Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H, Lockwood D, Lazzara R, Po SS. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2015 Mar 10;65(9):867-75.
7. Huang B, Yu L, Scherlag BJ, Wang S, He B, Yang K, Liao K, Lu Z, He W, Zhang L, Po SS, Jiang H. Left renal nerves stimulation facilitates ischemia-induced ventricular arrhythmia by increasing nerve activity of left stellate ganglion. J Cardiovasc Electrophysiol. 2014 Nov;25(11):1249-56.
8. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev-Prokophiev A, Zheleznev S, Shipulin V, Salakhutdinov N, Lomivorotov VV, Karaskov A, Po SS, Steinberg JS. Botulinum toxin injection in epicardial fat pads can prevent recurrences of atrial fibrillation after cardiac surgery: results of a randomized pilot study. J Am Coll Cardiol. 2014;12;64(6):628-9.
9. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation: A Randomized Clinical Trial. J Am Coll Cardiol. 2013, 62(24):2318-25.
Paul H Wooley
Kardiatonos Inc., USA
Title: A novel model of atherosclerosis in mice
Time : 12:00-12:30

Biography:
Paul H Wooley is the Chief Scientific Officer at Kardiatonos Inc., USA. He completed his PhD in Immuno-genetics at Guys Hospital Medical School. He was a Mayo Clinic Fellow and a Professor at Wayne State University, Wichita State University and KU Medical School, USA.
Abstract:
Porphyromonas gingivalis have been found in atherosclerotic lesions in humans. We developed a murine model of atherosclerosis using a 60% fat diet and gavage of PCB-77 and/or atherosclerotic lesions in humans. We developed a murine model of atherosclerosis using a 60% fat diet and gavage of PCB-77 and/or P. gingivalis. The model was assessed by histological examination of the aortic sinus, and ELISA assays for biomarkers of damage to the endothelial glycocalyx. C57/BL6 mice were raised from six weeks on either regular diet or a 60% fat diet. PCB-77 and/or P. gingivalis (ATCC 33277) suspensions were administered by oral gavage and mice sacrificed on days 10, 15 or 20. Frozen sections were cut through the aortic valve and analyzed for the presence and size of plaques, amount of fibrous tissue and inflammation. ELISA test kits measured thrombin-anti-thrombin complex, anti-thrombin III, total plasminogen activation inhibitor-1, syndecan 1, heparan sulfate and hyaluronan synthase 1. Animals receiving one PCB-77 gavage and one bacterial gavage demonstrated a low level of fibrous tissue and fat staining with a moderate level of inflammation. Fat staining was observed in foam cells in the artery walls of two sections in the aortic sinus with cholesterol inclusions. Most mice receiving three gavages of PCB-77 showed a moderate level of fat staining with low inflammation. Significantly higher levels of PAI-1 in mice were receiving one PCB-77 gavage and one bacterial gavage than in the control group. Levels of heparan sulfate detected in mice receiving three gavages of PCB-77 were significantly higher than in the control group. The levels of syndecan 1 and hyaluronan synthase 1 in the mice receiving three gavages of PCB-77 were significantly higher than in controls. The data show that administration of PCB and P. gingivalis to mice fed a high fat diet resulted in pathological features and biomarker abnormalities indicative of the presence of cardiovascular disease.
Image:
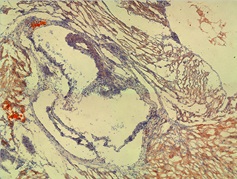
Figure shows an area of foam cells in the wall of the sinus containing lipid staining in mice receiving two gavages of PCB-77 and one gavage of bacteria.
References.
1. Fukasawa, A., Kurita-Ochiai, T., Hashizume, T., Kobayashi, R., Akimoto, Y., & Yamamoto, M. (2012). Porphyromonas gingivalis accelerates atherosclerosis in C57BL/6 mice fed a high-fat diet. Immunopharmacology and immunotoxicology,34(3), 470-476.
2. Arsenescu, V., Arsenescu, R. I., King, V., Swanson, H., & Cassis, L. A. (2008). Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis.Environmental health perspectives, 116(6), 761.
Lingfang Zeng
King's College London, UK
Title: A histone deacetylase 7-derived 7-amino acid peptide acts as a phosphorylation carrier
Time : 12:30-13:00

Biography:
Lingfang Zeng has his expertise in Stem Cell Research in Vascular Biology field, specifically on Mechanotransduction, Epigenetic Modification and Alternative Splicing. He has done extensive investigation on HDAC3, HDAC7 and IRE1α/XBP1 signal pathway. His current research focuses on Alternative Translation. His group found that small open reading frame within so-called non-coding area or non-coding RNA could be translated to produce functional small peptides. In addition to ATG, they found that other codons could also initiate the translation to produce more small peptides.
Abstract:
Histone deacetylase 7 (HDAC7) belongs to the class II HDAC family and plays a pivotal role in the maintenance of endothelium integrity. There are eight splicing variants in mouse HDAC7 mRNAs. Within the 5’ terminal non-coding area of some variants, there exist some short open reading frames (sORFs). Whether these sORFs can be translated or resulting peptides play roles in cellular physiology remain unclear. In this study, we demonstrated that one sORF encoding a 7-amino-acid (7-aa) peptide could be translated in vascular progenitor cells (VPCs). Importantly, this 7-aa peptide (7A) could transfer the phosphate group from the phosphorylated Ser393 site of MEKK1 to the Thr145 site of 14-3-3γ protein. The phosphorylated 7A (7Ap) could then directly phosphorylate 14-3-3γ protein in a cell-free, in-gel buffer system. The adjacent histidine and proline residues are essential for the phosphate group reception and transfer. In vitro functional analyses revealed that 7A and 7Ap increased VPC self-renewal and migration and enhanced vascular endothelial growth factor (VEGF)-induced VPC migration and differentiation toward the endothelial cell (EC) lineage, in which MEKK1 and 14-3-3γ served as the upstream kinase and downstream effector, respectively. Knockdown of either MEKK1 or 14-3-3γ attenuated VEGF-induced VPC migration and differentiation. Exogenous 7Ap could rescue the effect of VEGF on the MEKK1 siRNA-transfected VPCs but not on the 14-3-3γ siRNA-transfected VPCs. In vivo studies revealed that 7A, especially 7Ap, induced capillary vessel formation in matrigel plug assays, increased re-endothelialization and suppressed neointima formation in the femoral artery injury model, and promoted foot blood perfusion recovery in the hind limb ischemia model by increasing Sca1+ cell niche formation. These results indicate that the sORFs within the non-coding area can be translated and that 7A may play an important role in cellular processes such as proliferation, migration and differentiation by acting as a phosphorylation carrier.
References:
1. Yang J, et al (2016) Analysis of histone deacetylase 7 alternative splicing and its role in embryonic stem cell differentiation toward smooth muscle lineage. Methods Mol Biol. 1436:95-108.
2. Zhou B, et al (2011). Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear β-catenin translocation. Arterioscler Thromb Vasc Biol. 31(11):2676-84
3. Zhang Let al (2010). Sp1-dependent activation of HDAC7 is required for platelet-derived growth factor-BB-induced smooth muscle cell differentiation from stem cells. J Biol Chem. 285:38463-72
4. Margariti A, et al (2010). Histone deacetylase 7 controls endothelial cell growth through modulation of beta-catenin. Circ Res. 106:1202-11.
5. Margariti A, et al (2009). Splicing of HDAC7 modulates the SRF-myocardin complex during stem-cell differentiation towards smooth muscle cells. J Cell Sci. 122:460-70.
Mridula Dhakad
Saudi German Hospital, Dubai
Title: Newer treatments in heart failure
Time : 14:00-14:30

Biography:
Mridula Dhakad is a senior interventional Cardiologist with more than 25 years of experience in Interventional and Clinical Cardiology. She has actively participated in many national and international conferences. She has been extensively involved in treating many complex and critical cardiac patients. She has received her interventional training at well reputed large public and private hospitals. She did her fellowship in Interventional Cardiology at a world renowned large volume centre at Paris. She was instrumental in establishing Cardiology department at various reputed institutions. She is an active member of European Society of Cardiology and a life member of Cardiology.
Abstract:
Fight against reducing the disability in heart failure patients is on and tremendous advancements in management of such patients with low ejection fraction over past few decades has improved the prognosis. Wealth of research data is available on this, wherein the scientists have delved deep into studying the apoptosis of myocytes and how the failing cells can be brought back into function by targeting the maladaptive neurohormonal pathways. Across the spectrum of the heart failure patients, the following range of pharmacotherapy prescribed over the past years has definitely lowered the hospital admission rates and prolonged survival but still the readmission rates are high and financial burden on the healthcare systems are alarming–diuretics, renin-angiotensin-aldosterone system inhibitors, B-blockers, digoxin and vasodilators being the mainstay of therapy. The discovery of two new classes of drugs has marked a new beginning in the care of chronic heart failure patients. Ivabradine and sacubitril/valsartan seem to be promising agents in improving the functional capacity and quality of life, reducing the hospital readmission rates and mortality. They reduce the stress on the myocardium by reducing the heart rate and improve the blood flow by relaxing the vessels thereby improving clinical outcomes. End stage heart failure patients still queue up for heart transplantation worldwide but lack of available donors necessitates them to use ventricular assist devices (like LVAD) as a bridge therapy in capable centers. ECMO also has been used for short term circulatory support. The key focus in this talk will be on novel drug treatment for systolic heart failure.
Jianrui Song
University of Michigan, USA
Title: Myeloid-specific IL4 receptor alpha knockout increases adverse cardiac remodeling post myocardial infarction
Time : 14:30-15:00

Biography:
Jianrui Song is a PhD candidate in Cell and Developmental Biology at University of Michigan. She has received her Master’s Degree from China in 2009, where she worked on “The role of autophagy in hypoxic microenvironment and chemo- and radiotherapy in liver cancer”. As a PhD candidate, she is pursuing training in the field of Cardiovascular Disease and Immunology. She is working on how myeloid cells participate in the response of cardiac ischemia, the formation of infarct and the post infarct response, and also in determining how altering myeloid (especially macrophage) phenotype by IL4Ra can affect these responses in order to identify processes that can be targeted for beneficial effect. She is supported by the Bradley Merrill Patten Research Fellowship and by an American Heart Association Pre-doctoral Fellowship.
Abstract:
Myocardial infarction (MI) elicits inflammatory reactions that are orchestrated by a wide range of immune cells including macrophages. Reprogramming macrophages towards a resolving and reparative phenotype is a potential therapeutic approach for MI. However, how to modify macrophages in order to improve cardiac injury is not clear. Interleukin 4 receptor alpha (IL4Ra) activation is one of the major alternative activated macrophage (also names M2 macrophage) inducers, so IL4Ra is a potential macrophage modifier. Here, we knocked out IL4Ra from myeloid cells to determine how IL4Ra signaling is involved in cardiac remodeling post MI. Myeloid-specific IL4Ra knockout (MyIL4RaKO) did not show significant change in cardiac hypertrophy and interstitial fibrosis. There was decreased infarct size at one week post MI, but by three weeks there was no significant difference in infarct size. We saw significantly increased infarct thickness and perivascular fibrosis at three weeks, indicating the involvement of IL4Ra signaling in cardiac remodeling. Importantly, along with these significant changes in cardiac remodeling, MyIL4RaKO mice showed significantly decreased cardiac function at three weeks post MI. IL4Ra signaling was significantly inactivated in macrophages; however no change was shown in macrophage polarization in heart tissues post MI. In conclusion, IL4Ra signaling in myeloid cells is critical in maintaining proper cardiac remodeling and cardiac function post MI, which suggests the potential of modifying macrophages through enhancing IL4Ra signaling as a therapeutic strategy for MI.
References :
1.Manabu Shiraishi, Yasunori Shintani, Yusuke Shintani, Hidekazu Ishida, Rie Saba, et al, and Ken Suzuki. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016 Jun 1;126(6):2151-66.
2.Janine Woytschak, Nadia Keller, Carsten Krieg, Daniela Impellizzieri, Robert W. Thompson, et al, and Onur Boyman. Type 2 Interleukin-4 Receptor Signaling in Neutrophils Antagonizes Their Expansion and Migration during Infection and Inflammation. Immunity. 2016 Jul 19;45(1):172-84.
3. Johanna A. Knipper, Sebastian Willenborg, Ju¨ rgen Brinckmann, Wilhelm Bloch, Tobias Maaß, et al, and Sabine A. Eming.Interleukin-4 Receptor alpha Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015 Oct 20;43(4):803-16.
4.Slava Epelman, Kory J. Lavine, Anna E. Beaudin, Dorothy K. Sojka, Javier A. Carrero, Boris Calderon, Thaddeus Brija, Emmanuel L. Gautier, Stoyan Ivanov, Ansuman T. Satpathy, Joel D. Schilling, et al, and Douglas L. Mann. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014 Jan 16;40(1):91-104.
5.Michael G. Usher, Sheng Zhong Duan, Christine Y. Ivaschenko, Ryan A. Frieler, Stefan Berger, Günther Schütz, Carey N. Lumeng, and Richard M. Mortensen. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010 Sep;120(9):3350-64.
Niraj Khatri Sapkota
Chitwan Medical College- Tribhuvan University, Nepal
Title: Waist circumference is strong predictor of hypertension in male
Time : 15:00-15:30

Biography:
Niraj Khatri Sapkota has completed his PhD in Molecular Physiology applications to pharmacology at the age of 32 years from Zhejiang University, China, one of the Thomson Reuters and Elsevier best ranked university of the world; he is now working as an Associate Professor in the Department of Physiology in Chitwan Medical College affiliated to Tribhuvan University, Nepal. He is an active researcher and academician of his country, Nepal. He has published more than 50papers both original and review papers as a single author or with collaboration in reputed international journals and is serving as a reviewer, advisory and editorial board member and Editor of more than 30 international reputed journals.
Abstract:
Hypertension is one of the cardiovascular variables raised state, systolic and diastolic blood pressure are its parameters that can be easily measured, alteration of blood pressure is commonly observed in different condition, but persistent elevated state is one of the alarming remark of the cardiovascular biology, therefore status of blood pressure in the adult in different anthropometric measures are adopted but especial focus is given to waist circumference at different anatomical site said to be one of the indicator of adiposity depots that is associated with cardio metabolic risk.
Aims: Hence, this study aims to find whether addition of waist circumference (WC) to body mass index (BMI; kg/m2) play additive or strong independent role in predicting health risk than does BMI alone.
Methods: A community based cross sectional study was conducted by incorporating total of substantial number (more than 100) of subjects in the data who were male only older than 25 years, non smokers, non alcoholic, didn’t have history of taking any type of medication, non vegetarian with normal physical activity and were residents in the urban and rural areas throughout, were included in the present study. Waist circumference referenced to umbilicus measured by non tensile and non flexible measuring tape and at the mean time height and weight were also recorded by standard device in order to calculate BMI and blood pressure was measured by Aneroid sphygmomanometer of the respective subject subsequently data analysis was made by using SPSS to compare the BMI and Waist circumference relationship with blood Pressure independently to identify their relationship with hypertension.
Results: Keeping few exceptional aside, Both BMI and Waist Circumference exhibited positive association with blood pressure, while the waist circumference was more strongly associated with hiking of blood pressure and also BMI is not always the relating parametric tool to metabolic disease as was conventionally considered.
Conclusion
The result and analytical data showed that (P<0.05) there is significant strong correlation of blood pressure with waist circumference comparatively more than BMI thus WC alone can significantly predict the co-morbidity therefore this study approach to suggest and hints to follow as a routine task for measuring Waist circumference while taking inference for diagnosing hypertension risk at least in male.
Reem Kayyali & Aliki Peletidi
Kingston University London, UK
Title: Contribution of pharmacists in cardiovascular disease prevention journey
Time : 15:30-16:00

Biography:
Reem Kayyali completed her Pharmacy Degree at Nottingham University; MSc in Bio-pharmacy at King’s College London and; PhD in the Management of Thalassaemia. She was awarded the Maplethorpe fellowship for two years at King’s College London. After that, she worked for four years as a Research Fellow at University College London Medical School. In 2006, she joined Kingston University and now is an Associate Professor and the Director of Research and Enterprise in the School of Life Sciences, Pharmacy and Chemistry. Her current research interests are focused on “Medicines optimization, public health and technology enabled care”.
Aliki Peletidi completed her Pharmacy Degree at Kingston University London. Currently, she is pursuing her PhD about the role of pharmacists in cardiovascular disease prevention.
Abstract:
Statement of the Problem: Cardiovascular disease (CVD) is the primary cause of death worldwide causing 29.6% in 2010. In the UK, CVD was the second cause of mortality, responsible for 27% deaths in 2014 and 48% of all deaths in Greece in 2012. CVD is a result of risk factors such as hypertension, dyslipidemia, overweight/obesity, stress, smoking, physical inactivity and diabetes. CVD prevention consists of two stages: Primary and secondary. Pharmacists, one of the most accessible healthcare professionals, do not have a clear role in the existing pathways. In our view, there are three prevention stages: Primary, secondary and tertiary. The primary prevention constitutes enhancing awareness and initial screening of the public for CVD risk factors (obesity, lack of exercise, alcohol misuse and tobacco use) in order to estimate their CVD risk. Pharmacists could offer services such as weight management, alcohol brief intervention, smoking cessation and health checks. Secondary prevention is the management of post-diagnosis risk conditions (diabetes, hypertension and dyslipidemia) in order to prevent the incidence of a CVD disease. At this stage, pharmacists could provide services with a view to optimize medicines to improve medication adherence and to offer lifestyle advice. Tertiary prevention is the linkage between prevention and treatment for those who already suffered a CVD episode with a view to decreasing the risk of a secondary episode or mortality.
Methodology & Theoretical Orientation: A qualitative study utilizing semi-structured interviews with UK and Greek pharmacists was undertaken to explore their current and future roles in CVD prevention. The conceptual framework used was based on the results of the literature search conducted on the pharmacist's role in CVD risk factors and conditions. The interview schedule, which included 28 questions in three sections, was designed based on the proposed CVD prevention pathway. Purposive sampling was used, obtaining 40 participants in total for both countries.
Results: The analysis identified the following themes; role recognition and priority services. Both UK and Greek pharmacists played a role in secondary prevention, after risk conditions have been diagnosed. They are eager to monitor patients’ blood pressure or blood glucose when necessary, and provide lifestyle advice when they dispense the prescribed medications to them. Furthermore, in Greece it was identified that pharmacists had an appetite to initiate CVD prevention services. Pharmacists were willing to start a weight management programme to help their clients to reduce their CVD risk. Therefore, a weight management service was designed and is currently being evaluated.
Conclusion & Significance: This study identified UK and Greek pharmacists’ perspectives on their role in CVD prevention. Pharmacists’ role is mainly focused in secondary and less in primary and tertiary CVD prevention. However, in Greece, pharmacists recognized a potential role in primary prevention and early screening of CVD. There is a need of more work targeted in tertiary prevention to reduce complications and the chance of a secondary or a third CVD episode as well as premature mortality.
References :
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128.
2. Townsend N, Bhatnagar P, Wilkins E, Wickramasinghe K, Rayner M. Cardiovascular disease statistics 2015, British Heart Foundation: London.
3. World Health Organization. Greece: Noncommunicable Diseases (NCD) Country Profiles. Available from: http://www.who.int/nmh/countries/grc_en.pdf [Accessed: 16th October 2016].
4. Peletidi A, Nabhani-Gebara S, Kayyali R. Smoking Cessation Support Services at Community Pharmacies in the UK: A Systematic Review. Hellenic Journal of Cardiology 2016; 57: 7-15.
5. Yusuf S, Hawken S, Ounpuu T, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case control study. The Lancet. 2004; 364: 937–52.
6.Hobbs F. Cardiovascular Disease: Different Strategies for Primary and Secondary Prevention?. Heart 2004; 90:1217–1223.
Andrey V Kuznetsov
Innsbruck Medical University, Austria
Title: Cytoskeleton and regulation of mitochondrial function: The role of beta-tubulin II and plectin
Time : 16:15-16:45

Biography:
Andrey V Kuznetsov has worked on the problems of delicate mechanisms of energy metabolism in the heart and their changes under pathophysiological conditions. He has studied mitochondrial function and regulation in normal cells and in various pathological states, in particular in the field of mitochondrial cardiac system, mitochondrial interactions with other intracellular structures and systems, performing analysis of mitochondria in situ and in vivo. Also, he studied the roles of mitochondrial damage in ischemia reperfusion injury, apoptosis, calcium homeostasis, oxidative stress, mitochondrial ROS production, cardiac and muscle diseases with imaging of mitochondria and mitochondrial function, mitochondrial dynamics (fission, fusion, motility) in various living cells. He has extensive knowledge in cardiac bioenergetics and mitochondrial physiology and experience in optical imaging (fluorescent, two-photon) of single cell and single mitochondria, imaging of mitochondrial Ca2+, ROS, membrane potential, mitochondrial dynamics for basic science and in clinically oriented studies.
Abstract:
The control of mitochondrial function is a cardinal issue in the field of cardiac bioenergetics, and the analysis of mitochondrial regulations is central to basic research and in the diagnosis of many diseases. Interaction between cytoskeletal proteins and mitochondria can actively participate in mitochondrial regulation. Potential candidates for the key roles in this regulation are the cytoskeletal proteins plectin and tubulin. Analysis of cardiac cells has revealed regular arrangement of β-tubulin II, fully co-localized with mitochondria. β-tubulin IV demonstrated a characteristic staining of branched network, β-tubulin III was matched with Z-lines and β-tubulin I was diffusely spotted and fragmentary polymerized. In contrast, HL-1 cells were characterized by the complete absence of β-tubulin II. Comparative analysis of cardiomyocytes and HL-1 cells with cardiac phenotype revealed a dramatic difference in the mechanisms of mitochondrial regulation. In the heart, colocalization of β-tubulin isotype II with mitochondria suggests that it can participate in the coupling of ATP-ADP translocase (ANT), mitochondrial creatine kinase and VDAC. This mitochondrial supercomplex is responsible for the efficient intracellular energy transfer via the phosphocreatine pathway. We found also that in skeletal muscle of plectin knockout mice, mitochondrial content was reduced; mitochondria were aggregated in sarcoplasmic and subsarcolemmal regions, and were no longer associated with Z-disks. Our results show that the depletion of distinct plectin isoforms (P1b and P1d) affects mitochondrial network organization and function in different ways. Existing data suggest that cytoskeletal proteins may control the VDAC, contributing to the regulation of mitochondrial and cellular physiology.
References:
1. Bagur R, Tanguy S, Foriel S, Grichine A, Sanchez C, Pernet-Gallay K, Kaambre T, Kuznetsov AV, Usson Y, Boucher F, Guzun R. (2016) The impact of cardiac ischemia/reperfusion on the mitochondria-cytoskeleton interactions. Biochim. Biophys. Acta. 1862(6):1159-71.
2. Winter L, Kuznetsov AV, Grimm M, Zeöld A, Fischer I, Wiche G. (2015) Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle. Human Molecular Genetics. 24(16):4530-44.
3. Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. (2014) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta. 1853(2):276-284.
4. Varikmaa M, Bagur R, Kaambre T, Grichine A, Timohhina N, Tepp K, Shevchuk I, Chekulayev V, Metsis M, Boucher F, Saks V, Kuznetsov AV, Guzun R. (2014) Role of mitochondria-cytoskeleton interactions in respiration regulation and mitochondrial organization in striated muscles. Biochim. Biophys. Acta. 1837(2):232-45.
5. Tepp K, Mado K, Varikmaa M, Klepinin A, Timohhina N, Shevchuk I, Chekulayev V, Kuznetsov AV, Guzun R, Kaambre T. (2014) The role of tubulin in the mitochondrial metabolism and arrangement in muscle cells. J. Bioenerg. Biomembr. 46:421-434.
6. Javadov S, Kuznetsov AV. (2013) Mitochondria: the cell powerhouse and nexus of stress. Frontiers in Physiology. Vol. 4, article 207.
7. Kuznetsov AV, Javadov S, Guzun R, Grimm M, Saks VA. (2013) Cytoskeleton and regulation of mitochondrial function: the role of beta-tubulin II. Frontiers in Physiology. Vol. 4, article 82.
Ahmed N Ghanem
Retired Consultant Urologist, Egypt
Title: Volumetric overload shocks in the pathoetiology of the transurethral resection prostatectomy syndrome and acute dilution hyponatraemia
Time : 16:45-17:15

Biography:
Abstract:
The transurethral prostatectomy syndrome (TURS) is defined as severe vascular hypotension reaction that complicates endoscopic surgery as a result of massive irrigating fluid absorption causing severe acute dilution hyponatremia (HN) of <120 mmol/l. The vascular shock is usually mistaken for one of the recognized shocks and volumetric overload shock type 1 (VOS1) is overlooked making volumetric overload shock type 2 (VOS2) unrecognizable. In adults VOS1 is induced by the infusion of 3.5-5 liters of sodium-free fluids and is known as TURS or HN shock. VOS2 is induced by 12-14 liters of sodium-based fluids and is known as the adult respiratory distress syndrome. The most effective treatment for VOS1 and VOS2 is hypertonic sodium of 5%NaCl or 8.4% sodium bicarbonate. The literature on TURS is reviewed and the underlying pathoetiology is discussed. As Starling’s law for the capillary-interstitial fluid transfer, which underlies the principles of fluid therapy, proved wrong an alternative mechanism was found by studying the hydrodynamics of the porous orifice (G) tube akin to capillary. Incorporating the G tube in a chamber (C), representing the interstitial space surrounding a capillary, demonstrated a rapid dynamic magnetic field-like fluid circulation between the C and G tube lumen. The G-C phenomenon is autonomous having both filtration and absorption forces making a true replacement for Starling’s law in every tissue and organ of the body.
Sherif A S A Mansour
Ain Shams University, Egypt
Title: The value of preoperative administration of aminophylline as a cardio protective agent during coronary artery bypasses grafting
Time : 17:15-17:45

Biography:
Sherif A S A Mansour is a Consultant of Cardiothoracic Surgery in Ministry of Health Hospitals, in addition to working as a Surgeon in Royal Stoke Hospital, Stoke-on-Trent, UK. He works at Ain Shams University in Cairo, Egypt as a Senior Lecturer. He is subspecialized in Off Pump CABG, minimal invasive cardiac surgery with fantastic results and many contributions to various cardiac surgery centers in the Middle East, Asia and Africa. He has publications in the peer reviewed journals and contributions in some published cardiothoracic books with many contributions at international conferences across Europe and Asia. Recently, he started to explore the field of Robotic Heart Surgery with strong steps toward achieving his goal of mastering it.
Abstract:
Objectives: Myocardial ischemic reperfusion injury remains the most uncontrolled aspect of cardiac operations. This study aims at testing the hypothesis that aminophylline could serve as a potential myocardial protector against reperfusion injury during coronary artery bypass grafting (CABG).
Methods: A prospective, randomized, single blinded, placebo - controlled study involving 60 patients divided into two groups. Group A (30 patients) preoperatively received aminophylline, 200 mg orally per day for 3 days with a total dose of 600 mg. Group B (30 patients) was given placebo. All patients underwent uniform pre/intra/post-operative management. Assessment of cardiac markers (Troponin I) and cardiac enzymes were done before induction of anesthesia, after 30 min of aortic cross clamping and postoperatively (1, 24 and 48 hours). Preoperative concentration of aminophylline in serum was measured. Postoperative, 12 lead ECG after 48 hours and echocardiographic full study examination at the 6th day were examined.
Results: Total ICU stay hours and ventilation hours were statistically higher (p-value<0.05) in group B (67.20±21.28 and 16.47±8.76 hours, respectively) than in group A (46.87±14.46 and 12.20±3.56 hours, respectively). The need of inotropes in group A was lower than that in group B in the immediate postoperative period. However, Troponin I and cardiac enzymes in both groups were of no statistical significant difference (p-value>0.05).
Conclusions: Aminophylline may have beneficial role in CABG surgeries by decreasing the ventilation hours, need of inotropic support and total ICU stay hours. However, there was no relevant impact on cardiac marker and enzymes.
Hamid Amer
King Abdulaziz Hospital for National Guards, Saudi Arabia
Title: The significance of ECG changes during adenosine infusion as a stress agent for myocardial perfusion imaging (MPI) in predicting coronary artery disease (CAD)
Time : 17:45-18:15

Biography:
Hamid Amer is Nuclear Cardiology Board Certified and is an Associate Consultant in Nuclear Medicine at King Abdulaziz Hospital for National Guards, Saudi Arabia. He is involved in researches related to the Department of Nuclear Cardiology.
Abstract:
Purpose: The significance of electrocardiographic ECG changes during adenosine stress test is debatable. This study will further elaborate the significance of these changes in predicting the possibility of CAD.
Methods: This was a retrospective observational registry performed in a single center at the Kingdom of Saudi Arabia. The data were collected from the nuclear medicine database identifying all the reported Gated myocardial perfusion SPECT with adenosine stress tests between January 2013 and January 2014. The adenosine dose were fixed with all patients based on body weight and was given as a continuous infusion of 140 mcg/kg/min over a 6-minute period.
Results: There were 346 patients identified with cardiac nuclear scans in the pre-specified time frame who were subjected to adenosine stress scan. 152 of these patients were male accounting for 44% of the total population. Average age at the time of examination was 60.82±11.29 years. Patients were presented with one or more risk factors. Patients with base line abnormalities, past history of CAD with or without PCI or CABG and previous MI were excluded from the study. Ninety-eight patients (28%) were reported as positive for CAD, 40 patients were reported with ischemic ECG changes (12%) during adenosine infusion, 23 patients who have ischemic changes were positive on MPI, while 17 patients who have positive ECG had a negative MPI. Odd Ratio (OR) was 4.16, 95% C.I. was (2.11-8.18). Fisher Exact test was applied and showed a P value of <0.01. No reported case of asystole, myocardial infarction or complete heart block in this study period.
Conclusions: Our study showed that the probability of a positive MPI is 4 times higher with positive ECG ischemic changes during adenosine stress test and consequently these ECG ischemic changes should be taken in consideration in the final report.
- Congestive Heart Failure | Research on Cardiology | Cardiomyopathy | Hypertension | Coronary Artery Bypass Graft Surgery
Location: London, UK

Chair
Mridula Dhakad
Saudi German Hospital, UAE

Co-Chair
Barbara Petracci
Policlinico San Matteo IRCCS, Italy
Session Introduction
Barbara Petracci
Policlinico San Matteo IRCCS, Italy
Title: Adaptive-ness and effective-ness: The two primary goals to achieve in CRT
Time : 11:25-11:55

Biography:
Barbara Petracci is a Medical Doctor in Department of Cardiology, University of Pavia, Italy. She is mainly involved in the clinical activity of the Pacing and Electrophysiology Unit of the Department of Cardiology of the IRCCS San Matteo Hospital. As first operator, she usually performs pacemaker and defibrillator implantation procedures, including devices for cardiac resynchronization therapy, and catheter ablation of cardiac arrhythmias, including atrial fibrillation and ventricular tachycardia ablation. She is an experienced operator in laser catheters extraction. She is a member of the Italian Society of Pediatric Cardiology and Congenital Cardiomyopathy (SICP), a member of the Italian Association of Hospital Cardiologist (ANMCO), a member of the European Heart Rhythm Association (EHRA) and a member of the Italian Association of Arrhythmology and Cardiac Pacing.
Abstract:
Cardiac Resynchronization Therapy (CRT) is known as a highly effective therapy in advanced heart failure patients with cardiac dissinchrony. However, still one third of patients do not fully respond to CRT. Among the many contributors for the high rate of non-responders, the lack of procedures dedicated to CRT device settings optimization is known as one of the most frequent. On the other side, the echocardiography optimization is not widely used in the real world of CRT follow up visits. Thus, device-based techniques have been developed to by-pass the need of repeated echo evaluations to optimize CRT settings. There are multiple drivers of non-response. Common factors are AV/VV timing, reduced BiV pacing, LV lead placement, presence of arrhythmias, appropriate patient selection, patient compliance and presence of comorbidities. The current challenge facing practitioners is to maximize the rate of patients who respond to CRT and the magnitude of the response. A very particular and important subgroup of HF population with CRT includes the patients with AF since the optimal use of CRT in this cluster remains uncertain. The current area of interest achieving these goals includes the tailoring patients’ selection, the individualizing LV placement and, in particular, the application of new technologies and algorithms for CRT delivery in optimal fashion, reducing inappropriate shocks incidence and optimizing device longevity.
Qiuping Zhang
King's College London, UK
Title: Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis
Time : 11:55-12:25

Biography:
Qiuping Zhang has her expertise in “Nesprin family research in Cardiovascular Biology field, specifically on understanding the complex roles of nesprin-1 and -2, family members of multi-isomeric scaffolding proteins, in regulating normal and pathological processes in the heart and muscle”. She has studied extensively the role of nesprins in myoblast differentiation and function as well as mutations in nesprin-1 or -2 in associations with Emery Dreifuss muscular dystrophy and dilated cardiomyopathy. Her current research focuses on “Defining the roles of nesprins in cardiac cell function by determining the functional significance of novel nesprin-1 and -2 mutations on the linker of nucleoskeleton-and-cytoskeleton complex that connects the nuclear envelope to the actin cytoskeleton, and also identifying novel roles for nesprins in the sarcomere”. This may reveal novel pathways that contribute to the development of cardiac cell dysfunction and cardiomyopathy.
Abstract:
Nesprins-1 and -2 are highly expressed in skeletal and cardiac muscle and together with SUN (Sad1p/UNC84)-domain containing proteins and lamin A/C form the linker of nucleoskeleton and cytoskeleton (LINC) bridging complex at the nuclear envelope (NE). Mutations in nesprin-1 and -2 have previously been found in patients with autosomal dominant Emery-Dreifuss muscular dystrophy 4 (AD-EDMD 4, OMIM 612998) and 5 (AD-EDMD5, OMIM 612999) as well as dilated cardiomyopathy (DCM). In this study, three novel rare variants (R8272Q, S8381C and N8406K) in the C-terminus of the SYNE-1 gene (nesprin-1) were identified in 7 DCM patients by mutation screening. Expression of these mutants caused nuclear morphology defects and reduced lamin A/C and SUN2 staining at the NE. GST-pull down indicated that nesprin-1/lamin/SUN interactions were disrupted. Nesprin-1 mutations were also associated with augmented activation of the ERK pathway in vitro and in hearts in vivo. During C2C12 muscle cell differentiation, nesprin-1 levels are increased concomitantly with kinesin light chain (KLC-1/2), and immunoprecipitation and GST-pull down showed that these proteins interacted via a recently identified LEWD domain in the C-terminus of nesprin-1. Expression of nesprin-1 mutants in C2C12 cells caused defects in myoblast differentiation and fusion associated with dysregulation of myogenic transcription factors and disruption of the nesprin-1 and KLC-1/2 interaction at the outer nuclear membrane. These findings support a role for nesprin-1 in myogenesis and muscle disease, and uncover a novel mechanism whereby disruption of the LINC complex may contribute to the pathogenesis of DCM.
Sherif A S A Mansour
Ain Shams University, Egypt
Title: Benefits of Sildenafil to patients suffering from severe pulmonary hypertension secondary to mitral stenosis undergoing mitral valve replacement
Time : 12:25-12:55

Biography:
Sherif A S A Mansour is a Consultant of Cardiothoracic Surgery in Ministry of Health Hospitals, in addition to working as a Surgeon in Royal Stoke Hospital, Stoke-on-Trent, UK. He works at the Ain Shams University in Cairo, Egypt as a Senior Lecturer. He is subspecialized in Off Pump CABG, minimal invasive cardiac surgery with fantastic results and many contributions to various cardiac surgery centers in the Middle East, Asia and Africa. He shared in the establishment of more than one cardiac surgery center across Egypt with training of the staff and the surgical assistants and registrars. He also had publications in the peer reviewed journals and contributions in some published cardiothoracic books with many contributions at international conferences across Europe and Asia. Recently, he started to explore the field of Robotic Heart Surgery with strong steps toward achieving his goal of mastering it.
Abstract:
Introduction & Objective: Sildenafil (phosphodiesterase inhibitor type 5) has been successfully used to treat primary pulmonary hypertension. Mitral valve disease notably stenotic lesion is a long standing disease, in developing countries late detection results in increase association with severe secondary (PAH) pulmonary hypertension. Right ventricular (RV) failure is a leading cause of increased morbidity and mortality of patients with severe pulmonary hypertension undergoing mitral valve surgery. The study aims to confirm the effectiveness of preoperative oral sildenafil in decreasing the incidence of RV failure in this category of patients.
Methods: Eighty patients scheduled for mitral valve replacement surgery with severe PAH, RV systolic pressure (RVSP) ≥60mmHg were included in the study. Patients were randomized into two groups: Gr C - placebo (N=40), and Gr S - Sildenafil (N=40) with oral sildenafil 25 mg eight hourly for 48 h before surgery with the last dose given 25 mg in 10 ml via nasogastric tube after induction of anesthesia. In both group the need for inotropes was recorded and doses were titrated to achieve hemodynamic stability during and after cardiopulmonary bypass (CPB).
Results: Pulmonary artery pressure was significantly reduced in the sildenafil group. Ventilation time was less in the sildenafil group but without statically significant value, CPB time was significantly lower in the sildenafil group (p=0.05) and postoperative ITU stay was significantly lower (P<0.001) in sildenafil group. The requirements of inotropes notably dobutrex and milrinone were significantly more with placebo group compared to sildenafil group.
Conclusions: It is concluded that sildenafil is safe and effective in reducing severe pulmonary hypertension when given to patients prior to the mitral valve replacement surgery; which facilitated weaning from CPB.
Mridula Dhakad
Saudi German Hospital, UAE
Title: Interesting case presentation in Aortic Dissection
Time : 15:00-15:30

Biography:
Mridula Dhakad is a senior interventional Cardiologist with more than 25 years of experience in Interventional and Clinical Cardiology. She has actively participated in many national and international conferences. She has been extensively involved in treating many complex and critical cardiac patients. She has received her interventional training at well reputed large public and private hospitals. She did her fellowship in Interventional Cardiology at a world renowned large volume centre at Paris. She was instrumental in establishing Cardiology department at various reputed institutions. She is an active member of European Society of Cardiology and a life member of Cardiology.
Abstract:
Aortic dissection has varying presentations. This disease entity can mimic other acute emergencies posing a diagnostic dilemma. There should be high index of suspicion in diagnosing aortic dissection in patients of all ages. With newer advanced modalities of diagnosis and the risk score algorithms, the clinical outcome of an otherwise catastrophic disease has improved to a great extent. All the patients of collagen disease, bicuspid aortic valve, coarctation of aorta, uncontrolled HT and unexplained AR should be thoroughly evaluated. This interesting case of a 73 year old elderly gentleman presented with sudden onset chest discomfort radiating to the jaw with a short episode of sudden shortness of breath. The physical examination was unremarkable with stable hemodynamics and well felt peripheral pulses with no discrepancy. His D dimers were found to be markedly elevated with raised serum creatinine. The cardiac enzymes were normal. ECG was not indicative of myocardial ischemia. Echo showed minimal AR, mild anterolateral pericardial effusion with good LV systolic function and PA pressure of 32 mm Hg. He was hyper-tensed on medication with history of CVA 3 years ago and was on Warfarin since then. He remained stable hemodynamically for the initial 24 hours after admission, later he developed atrial fibrillation with controlled ventricular response. Owing to renal dysfunction, instead of pulmonary CT angiography, VQ scan was done to rule out pulmonary embolism. It revealed multiple perfusion defects in both lung fields. Simultaneous repeat echocardiography revealed increase in grade of AR to more than 1/4 and mild increase in PE with echogenicity seen within the effusion behind RV (possibly blood). CT chest was immediately performed which clinched the diagnosis of type A aortic dissection. He was immediately referred to the cardiac surgeon who did emergency surgical correction with successful recovery.
Amjed Eljaili
Betsi Cadwalader University Health Board, UK
Title: Left ventricular thrombus complicated by acute stroke due to consequence of cocaine abuse
Time : 15:30-16:00

Biography:
Dr Amjed Eljaili, MBBS October 2010, University of Al-Zaiem Al-Azhari, Sudan, currently practicing in UK , Wales deanery , foundation year-2 trainee, BCUHB, emergency department, Ysbyty Gwynedd, He attended several a cademic meetings, regionally and nationally, He has participated in various national work-shops, congress. participation and membership with British institute of Radiology, UK.
Abstract:
Cocaine abuse can cause acute and chronic cardiovascular complications which include, coronary artery spasm , aortic dissection, myocardial infraction, left ventricular dysfunction and thrombosis that can lead to fatal thromboembolic event. It has been well described in the literature that cocaine significanlty increases the risk of ischemic stroke in young adults within 24 hours of use. Interestingly the risk of cerebral infarct relating to acute cocaine comsuption is notably higher than the usuall well known stroke risk factors.
A 32 years old gentleman who is known to have history of cocaine abuse, presented with an acute stroke, myocardial infraction and significant large left ventricular thrombus which took place within short time window following cocaine abuse.
Niraj Khatri Sapkota
Chitwan Medical College- Tribhuvan University, Nepal
Title: Waist circumference is strong predictor of hypertension in male
Time : 14:15-14:45

Biography:
Niraj Khatri Sapkota has completed his PhD in Molecular Physiology applications to pharmacology at the age of 32 years from Zhejiang University, China, one of the Thomson Reuters and Elsevier best ranked university of the world; he is now working as an Associate Professor in the Department of Physiology in Chitwan Medical College affiliated to Tribhuvan University, Nepal. He is an active researcher and academician of his country, Nepal. He has published more than 50papers both original and review papers as a single author or with collaboration in reputed international journals and is serving as a reviewer, advisory and editorial board member and Editor of more than 30 international reputed journals.
Abstract:
Background
Hypertension is one of the cardiovascular variables raised state, systolic and diastolic blood pressure are its parameters that can be easily measured, alteration of blood pressure is commonly observed in different condition, but persistent elevated state is one of the alarming remark of the cardiovascular biology, therefore status of blood pressure in the adult in different anthropometric measures are adopted but especial focus is given to waist circumference at different anatomical site said to be one of the indicator of adiposity depots that is associated with cardio metabolic risk.
Aims: Hence, this study aims to find whether addition of waist circumference (WC) to body mass index (BMI; kg/m2) play additive or strong independent role in predicting health risk than does BMI alone.
Methods: A community based cross sectional study was conducted by incorporating total of substantial number (more than 100) of subjects in the data who were male only older than 25 years, non smokers, non alcoholic, didn’t have history of taking any type of medication, non vegetarian with normal physical activity and were residents in the urban and rural areas throughout, were included in the present study. Waist circumference referenced to umbilicus measured by non tensile and non flexible measuring tape and at the mean time height and weight were also recorded by standard device in order to calculate BMI and blood pressure was measured by Aneroid sphygmomanometer of the respective subject subsequently data analysis was made by using SPSS to compare the BMI and Waist circumference relationship with blood Pressure independently to identify their relationship with hypertension.
Results: Keeping few exceptional aside, Both BMI and Waist Circumference exhibited positive association with blood pressure, while the waist circumference was more strongly associated with hiking of blood pressure and also BMI is not always the relating parametric tool to metabolic disease as was conventionally considered.
Conclusion
The result and analytical data showed that (P<0.05) there is significant strong correlation of blood pressure with waist circumference comparatively more than BMI thus WC alone can significantly predict the co-morbidity therefore this study approach to suggest and hints to follow as a routine task for measuring Waist circumference while taking inference for diagnosing hypertension risk at least in male.
Arunkumar Arasappa
Sri Manakula Vinayagar Medical College and Hospital, India
Title: Establishing a cardiac programme in rural or semiurban places - Feasible or not?
Time : 16:15-16:45

Biography:
Arunkumar Arasappa is a Consultant Cardiothoracic Surgeon and is an Assistant Professor in the Department of Cardiothoracic Surgery at Sri Manakula Vinayagar Medical College and Hospital, Pondicherry. He started the Department of Cardiothoracic Surgery in this Medical College Hospital which is located in a village called kalitheerthalkuppam, Pondicherry, India. We have started this cardiothoracic program and open heart surgeries since April 2016 onwards till date. His team has successfully completed more than 90 open heart surgeries in a span of 10 months which includes beating heart CABG, On-Pump CABG, valvular heart surgeries and both adult and pediatric congenital heart surgeries.
Abstract:
To discuss the feasibility of starting and establishing a superspecialty department like cardiothoracic surgery in rural and semi-urban centers is a herculean task. We planned to setup a pilot project on how to start a superspecialty department in Rural and semiurban places and also to do open heart surgical procedures at an affordable cost. We discussed our plan with a medical college hospital in our locality and they helped us to start this within their premises with available infrastructure. We closely followed up the inpatient crowd of the hospital and secured cases from all fields of medicine and especially from cardiology department. We managed to maintain a registry of cases and categorized patients based on their symptoms and who needed surgery. Our next task of building the infrastructure was aimed and we procured materials necessary to start this program which included setting up of operation theatre, CT ICU and postoperative wards. Our other task of recruiting staffs for caring the patients was breathtaking and was managed successfully. After establishing this we started our first open heart procedure on April 2016 and continued from thereon. Today, we had crossed more than 90 open heart surgeries apart from vascular and thoracic surgeries. We have now established a center which can perform all cardiac surgery programs that are done at any standard hospitals or medical colleges in major cities. Today we proudly say that the task is well started and could be achieved with the help of a supporting team of dedicated doctors and paramedical staffs and equally supported with the necessary infrastructure by the hospital to cater the underprivileged people with the support of various schemes and insurance assistance.

Biography:
Abstract:
Statement of the Problem: Mitral valve can be accessed through left atrium or via inter-atrial septum. Although left atrium is the traditional approach, trans-septal approach gives better exposure in difficult cases. This retrospective study was designed to evaluate the safety, pitfalls and effectiveness of the extended vertical trans-septal approach for routine mitral valve exposure.
Methodology & Theoretical Orientation: It is a retrospective study of 1017 consecutive patients undergoing an isolated primary mitral valve procedure (repair, replacement) through a median sternotomy between the years 2000 and 2015 by eight different surgeons. Out of these 135 patients were operated by extended vertical trans-septal approach (EVTSA, group A) while 882 patients were accessed through traditional left atrial (LA, group B) approach via posterior inter-atrial groove.
Findings: There were 135 patients (M/F=56/79) in group A and 882 patients (M/F=398/484) in group B. Logistic euro score was significantly lower in EVTSA group (0.61 vs. 0.90 p=0.000001). In LA group, there were more patients with pre-operative TIA or stroke (94 vs. 6 p=0.005), and this difference was statistically significant. Cumulative cross clamp time was 82 (44-212) minutes (EVTSA) and 78 (30-360) minutes (LA) groups (p=0.271) while cardiopulmonary bypass time was 107 (58-290) and 114 (43-602) minutes (p=0.121). Post-operative blood loss was 415 ml (EVTSA) versus 427 (LA) ml (p=0.273). No significant difference was found in the incidence of post-operative atrial fibrillation (p=0.22) or heart block requiring permanent pacemaker (p=0.14).
Conclusion & Significance: Extended vertical trans-septal approach is safe and reproducible. It gives excellent exposure of the mitral valve. It is reasonable way to routinely expose mitral valve without significant increase in cross clamp time, post-operative arrhythmia, heart block or bleeding.
Sarwat Sultan
Pakistan
Title: Treating heart disease by managing marital conflict among female patients with CHD through cognitive behavioural couple therapy
Time : 17:15-17:45

Biography:
Sarwat Sultan is the Chairperson of the Department of Applied Psychology, BZU, Pakistan. She has done her PhD in Applied Psychology in 2009, and has completed her Post-doctorate from Curtin University, Australia in 2013. She is serving the Department of Applied Psychology, Bahauddin Zakariya University Multan since the last 15 years. She has contributed more than 75 research articles in national and international journals, has presented more than 60 papers in conferences at national and International levels, and has supervised more than 150 dissertations. She has one book published in Germany to her credit. She is a distinguished teacher having experience of research and psychological testing and interventions.
Abstract:
Statement of the Problem: The purpose of this study was to assess the effectiveness of cognitive behavioral couple therapy (CBCT) in treating heart disease by managing marital conflict among female patients with coronary heart disease.
Methodology: This study was completed using repeated measure design with a sample of 20 female cardiac patients approached at Institute of Cardiology Multan who were matched on the characteristics of age, education and economic class. This sample was then randomly assigned to the intervention (n=10) and control groups (n=10). CBCT was administered to the intervention group (n-10) while control group was on medication only. Data were collected on measures of Kansas Marital Satisfaction (KMS) Scale, Revised Dyadic Adjustment Scale (RDAS) and coronary heart disease symptoms check list (CHDS) from both groups before and after the administration of CBCT to intervention group. The data were analyzed by employing Kolmogorov-Smirnov test, Mann-Whitney U test and Wilcoxon W test.
Findings: Significant differences were found in pre and post scores on marital satisfaction scale, dyadic adjustment scale and symptoms of CHD for intervention group. The CBCT was found to be effective in increasing stability and satisfaction in the marital relationship of females. Female Patients after CBCT also reported a decrease in their symptoms of CHD.
Conclusion: From the present findings, CBCT seems very effective in resolving conflicts and decreasing severity of symptoms of CHD among female cardiac patients.
Recommendations: CBCT can be considered as practicable method in managing marital conflicts associated with CHD.
REFERENCES :
1: World Health Organization. Global Tuberculosis Report 2013. Geneva; 2013.
2: Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007 Dec 15; 370(9604):2030-43.
3: Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation. 2005 Dec 6; 112(23):3608-16.
4: Strang G, Latouf S, Commerford P, Roditi D, Duncan-Traill G, Barlow D et al. Bedside culture to confirm tuberculous pericarditis. Lancet. 1991 Dec 21-28; 338(8782-8783):1600-1.
5: Pandie S, Peter JG, Kerbelker ZS. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-γ in a high burden setting: a prospective study. BMC Medicine. 2014;12:101. doi:10.1186/1741-7015-12-101.
S M Tajdit Rahman
Green Life Hospital, Bangladesh
Title: Congenital heart diseases among the hospital live birth in Bangladesh: A retrospective study in national institute
Time : 17:45-18:15

Biography:
S M Tajdit Rahman is a Resident Thoracic Surgeon of National Institute of Bangladesh. He has completed his MBBS from Sir Salimullah Medical College, Bangladesh and has a fascination for research in cardiac and thoracic diseases. He is doing research under renowned professors and has a great achievement in extracurricular activities. He is the Convener of first ever biomedical conference for students in Bangladesh. He has attended more than 15 national and international conferences.
Abstract:
Congenital cardiac disease is not uncommon in daily medical practice. Many studies have been carried out worldwide, showing incidence variation in different parts of the world as 5-10/1000 live birth. In Bangladesh, a mere study was done in this respect. This retrospective study was conducted from the records preserved in hospital register, compiled by the author from pediatric cardiology and cardiac surgery department over a period of 4 years extends from 2010-2013 in National Institute of Cardio Vascular Diseases Hospital. 6520 cases of live births weighing more than 1500gm and age over 28th weeks of gestational period were recorded by clinical examination and echocardiography with color Doppler. This study showed that 196 babies out of 6520 live births had CHD as 30/1000 live births. Study also expressed that higher incidence of CHD was observed in preterm baby than full term baby. Among the congenital heart lesions, atrial septal defect, ventricular septal defect, Patent ductus arteriosus, Tetralogy of Fallot`s, TGA were commonest having 20.41%, 13.78%, 10.71%, 8.67% and 4.59%, respectively. 15.81% of the patients had other associated somatic anomalies among which down syndrome was the commonest (7.14%). CHD of various patterns deserves crucial challenge among the newborns for management in Bangladesh. Various factors like high maternal age, drugs intake, antenatal infection, family history, gestational DM, down’s syndrome, mother having SLE are related to these diseases. Moreover, appropriate research can be accomplished taking large relevant sample gathering from different tertiary medical college hospitals to reveal actual scenario to prevent and treat the diseases.
- Case Study on Cardiology | Research on Cardiology | Biomarkers and Enzymes for Heart Disease | Obesity and Heart | Cardiac Regeneration and Repair
Location: London, UK

Chair
Annamaria Vianello
Gabriele Monasterio Foundation, Italy
Session Introduction
Samer Ellahham
Sheikh Khalifa Medical City, UAE & Cleveland Clinic, USA
Title: The importance of multidisciplinary approach
Time : 09:30-10:00

Biography:
Samer Ellahham has served as Chief Quality Officer for Sheikh Khalifa Medical City, since 2009. In his role, he has led the development of a quality and safety program that has been highly successful and visible and has been recognized internationally by a number of awards. As Chief Quality Officer and Global Leader, he has focus on “Ensuring that that implementation of this best practices leads to breakthrough improvements in clinical quality and patient safety”. He is the recipient of the Quality Leadership Award from the Global Awards for Excellence in Quality and Leadership and the Business Leadership Excellence Award from the World Leadership Congress. He was nominated in 2015 for SafeCare magazine Person of the Year. He is Certified Professional in Healthcare Quality (CPHQ). He is a recognized leader in quality, safety, and the use of robust performance improvement in improving healthcare delivery.
Abstract:
In most countries worldwide, the number of patients with chronic heart failure (HF) is growing with 1-3% of the adult population suffering from this syndrome, rising to about 10% in the very elderly. In the near future, a large part of the worldwide population will suffer from heart failure and society will be faced with the consequences. On average, one in five patients is readmitted within 12 months, making heart failure one of the most common causes of hospitalization in people over 65 years of age. A multidisciplinary team approach involving several professionals with their own expertise is important in attaining an optimal effect. Physicians, nurses and other health care professionals are keys ensuring the delivery of evidence based care. Markers of clinical (in) stability, psychosocial risk factors, and issues related to patient mobility might be important indicators to determine which inter-professional service might be most effective for which patient. Current HF guidelines recommend that HF patients are enrolled in a multidisciplinary-care management program to reduce the risk of HF hospitalization. A multidisciplinary approach to HF may reduce costs, decrease length of stay, curtail readmissions, improve compliance and reduce mortality. An important limitation, however, is the substantial heterogeneity in both the terms of the models of care and the interventions offered, including: clinic or community-based systems of care, remote management and enhanced patient self-care. Conventional trials that randomize individual patients may not be the best way to test the effect of a service; novel approaches, such as the cluster randomized controlled trial, may be superior. It is unlikely that any one approach is optimal. The best form of care might seek to compensate for the weaknesses of each approach by exploiting their strengths. A strong HF cardiology lead, supported by primary care physicians, nurse specialists and pharmacists in the hospital and community with the ability to offer patients remote support might offer the best service. Key to the success of multidisciplinary HF programs may be the coordination of care along the spectrum of severity of HF and throughout the chain-of-care delivered by the various services within the healthcare system. Further research is warranted to identify the most efficacious multidisciplinary approaches to HF.
Learning Objectives: Objectives are to: Define multidisciplinary approach to HF; examine the literature role and recommendations of multidisciplinary in HF and; identify barriers to optimal models of multidisciplinary approach to HF.
Annamaria Vianello
Gabriele Monasterio Foundation, Italy
Title: A case of misdiagnosed cardiomyopathy with rising plasma MMP9 long before sudden cardiac arrest
Time : 10:00-10:30

Biography:
Annamaria Vianello completed her Graduation at Medical School, Pisa University where she obtained Specialization both in Internal Medicine and in General Medicine. She completed her PhD in Medical Physiopathology and Pharmacology and in Normal and Pathological Morphology of Cells and Tissues; a second-level Short Specialization Degree in Clinical Pharmacological Research and; second-level Short Specialization Degree in Hypertrophic Cardiomyopathies at Pavia University. She worked as Fellow in Department of Internal Medicine and Sport Medicine, Pisa University. Her research interests include Athlete’s Heart Remodeling, Failing Heart Remodeling and Cardio-Renal Remodeling. She is currently coordinating a Tele-Health Project for out-of-hospital care of chronic heart failure. She is an Author of original papers dealing with the clinical role of circulating and tissue profiles of inflammatory biomarkers, MMPs and TIMPs in different models of heart remodeling such as Athlete’s Heart, Valvular Heart Disease, Cardio-Renal Failure and Chronic Heart Failure.
Abstract:
Introduction: Out-of-hospital cardiac arrest in the absence of structural heart disease is rare and can be due to subclinical cardiomyopathy or primary electrical disorders. Adverse collagen remodeling may occur in cardiac arrest patients; MMP-9 is proven to help risk stratification.
Clinical Case Description: In 2005, R.R, a 35 year old metal-worker, surprisingly began to show high values of MMP9. TIMPs, MMP2, NT-pro-BNP and all the other laboratory tests, abdominal echography, electrocardiogram and echocardiography were within the reference range. 10 years later, R.R suddenly collapsed at home; his wife promptly began a successful cardiac massage, supported by territorial tele-emergency network. R.R was admitted to the intensive unit with frequent ventricular extrasystoles, left ventricular dysfunction. Medical history of his family includes sudden death of two paternal uncles; mother with Takotsubo syndrome. Unremarkable clinical examination and electrocardiogram showed no biochemical evidence of acute myocardial infarction; CRP elevation. Un-obstructed coronary arteries on coronary were revealed in angiography. Chest X-ray showed mild interstitial fibrosis. There were no signs of arrhythmogenic cardiomyopathy on Cardio-NMR.
Discharge Diagnosis: Discharge diagnosis was: “Resuscitated cardiac arrest, in subject without evidence of structural heart disease, treated with ICD bicameral system”.
Reviewing Clinical Information & Diagnosis: Familial histories of sudden death, mild endocardial irregularities, left ventricle enlargement, reduced ejection fraction, low EKG voltage are suggestive of familial cardiomyopathy. Welding, chest X-ray fibrosis, RCP is suggestive of welder’s lung. Then, reformulated diagnosis: Resuscitated cardiac arrest in sub-clinical familial cardiomyopathy, chronically subjected to professional cardio-toxic damage.
Conclusions & Implications for Clinical Practice: R.R lethal arrhythmia was firstly considered as “idiopathic” in nature, since process laboratory and radiology testing were not analyzed properly. Interestingly, long before cardiac arrest, MMP9 had begun to rise, announcing a cardio-pulmonary adverse remodeling. The case of R.R teaches us that early biomarkers such as MMP9, correct and timely diagnosis, tele-medicine and emergency services network could really save lives of patients at high risk of sudden death.
Image:
Figure 1: Low EKG voltage on peripheral leads
References:
1. Flevari P, Theodorakis G, Leftheriotis D, Kroupis C, Kolokathis F, Dima K, Anastasiou-Nana M, Kremastinos D (2012) Serum markers of deranged myocardial collagen turnover: their relation to malignant ventricular arrhythmias in cardioverter-defibrillator recipients with heart failure. Am Heart J 164 (4):530-537.
2.Turkdogan KA, Zorlu A, Guven FM, Ekinozu I, Eryigit U, Yilmaz MB (2012) Usefulness of admission matrix metalloproteinase 9 as a predictor of early mortality after cardiopulmonary resuscitation in cardiac arrest patients. Am J Emerg Med 30(9): 1804-1809.
3. Hästbacka J, Tiainen M, Hynninen M, Kolho E, Tervahartiala T, Sorsa T, Lauhio A, Pettilä V (2012) Serum matrix metalloproteinases in patients resuscitated from cardiac arrest. The association with therapeutic hypoyhermia. Resuscitation 83(2): 197-201.
4.Fang AC, Cassidy A, Christiani DC (2010) Systematic Review of Occupational Exposure to Particulate Matter and Cardiovascular Disease. Int J Environ Res Public health 7: 1773-1806.
5.Schiff GD, Hasan O, Kim S, Abrams R, Cosby K, Lambert BL, Elstein AS, Hasler S, Kabongo ML, Krosnjar N, Odwazny R, Wisniewski MF, McNutt RA (2009) Diagnostic error in medicine: analysis of 583 Physician-Reported Errors. Arch Inter Med 169(20): 1881-1887.
Prashant Tarakant Upasani
Metro Hospital & Heart Institute, India
Title: Advances in management of heart failure with emphasis on Angiotensin receptor neprilysin inhibitor (ARNI)
Time : 10:45:11:15

Biography:
Prashant Tarakant Upasani completed his DM (Cardiology) at All India Institute of Medical Sciences, New Delhi in May 1994. After completing his DM (Cardiology), he worked as Associate Consultant Cardiologist at Apollo Hospitals, Chennai and Indraprastha Apollo Hospitals, New Delhi. He is presently working at Metro Heart Institute, Noida as Senior Consultant Interventional Cardiologist. He is actively involved in all diagnostic and therapeutic interventional procedures. He was awarded Fellowship of College of Chest Physicians in the field of Pediatric Cardiology. In December 2012, he was conferred with Fellowship of Cardio-logical Society of India. He has 99 publications both in national and international journals including text books on cardiology. He has presented more than 80 papers in various national and international conferences. He is also the Coordinator of DNB Cardiology program at Metro Hospital, Noida.
Abstract:
Heart failure (HF) is an increasingly common syndrome associated with high mortality and economic burden. A range of terms has been used to describe HF viz. chronic HF (CHF), acute HF (AHF), HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). Pathophysiology of HFrEF is complex. Landmark trails in patient with HFrEF include SOLVD-T, CIBIS-II, CHARM-Alternative and CHARM-Added, SHIFT, EMPHASISHF and PARADIGM-HF. Natriuretic peptides (NPs) are a group of hormones that have potent effects on sodium and fluid balance. Neprilysin is a zinc-dependent metallopeptidase that catalyses the degradation of various peptides. Inhibition of neprilysin increases bioavailability of NPs, bradykinin, and substance P, resulting in natriuretic, vasodilatory and anti-proliferative effects. LCZ696 is useful not only for the treatment of HF but also likely to be a useful antihypertensive drug. LCZ696 is a novel drug that comprises sacubitril (AHU377) and valsartan in a 1:1 molar ratio. It simultaneously inhibits neprilysin and blocks AT1 receptors. PARADIGM-HF is the first study to test the efficacy of LCZ696 on morbidity and mortality in patients with HFrEF. The trail was stopped early, according to pre-specified rules, after a median follow-up of 27 months, because the boundary for an overwhelming benefit with LCZ696 had been crossed. LCZ696 was associated with a significantly lower death from cardiovascular causes or first hospitalization (taken together-20% or separately 20-21%). Secondary endpoint viz. death from any cause, showed a 16% reduction (p<0.001). The superiority of LCZ696 over enalapril was not accompanied by important safety concerns. The LCZ696 group had a higher proportion of patients with non-serious angioedema, but not associated with an increase in serious angioedema.
Vladimir Ermoshkin
Russian New University, Russia
Title: The new theory of heart failure
Time : 11:15-11:45

Biography:
Vladimir Ermoshkin completed his Graduation in Physics department at Moscow State University in 1978. He has worked at Russian New University (RosNOU) as Physicist. He has published 10 articles on Cardiology in prominent magazines (Russian and English).
Abstract:
Aim:C делана попытка нового анализа м еханизм а An attempt to study the mechanism of heart failure.
Method: Information search in the literature, participate in conferences, discussions with Russian leading cardiologists.
Result: Having heart failure means that for some reason your heart is not pumping blood around the body as well as it used to. Heart failure can be a major manifestation of virtually all diseases of the heart, including coronary atherosclerosis, myocardial infarction, acquired valvular disease, congenital heart disease, arrhythmias and cardiomyopathy. Populationâ€based echocardiographic studies have demonstrated that more than 50% of participants with left ventricular systolic dysfunction (generally defined as LVEF <35–40%) have no symptoms or signs of heart failure. How to interpret this data? How the heart can pump insufficient blood to one person and sufficient amount of blood to another? Where is the logic? The reason according to my theory of CVD is that, near liver, arteriovenous anastomoses (AVA) or cascade AVA opens for longer time than optimal. Veins overflow begin mechano-induced arrhythmia. Opened AVA lead to stagnation of blood in the area of small pelvis and ankle/feet. The other reasons are stasis of microcirculations, weight gain, varicose veins, endometriosis, prostatitis, hemorrhoids, thrombosis, and cancer. The development of these diseases is accompanied in most cases with heart failure.
Conclusions: It is necessary to correct the glaring errors in cardiology which are existing from past 50-100 years. It is necessary to develop pulse wave suppressors and artificial anastomosis with an adjustable diameter hole.
1. British Heart Foundation is a registered Charity No. 225971. Internet resource. https://www.bhf.org.uk/heart-health/conditions/heart-failure
2. «ÐÐПЦССХ им. Ð.Ð. Бакулева» Минздрава РоÑÑии. Internet resource. http://www.bakulev.ru/cyclo/detail.php?ID=39219
3. Arend Mosterd, Arno W Hoes Clinical epidemiology of heart failure Journal List Heart v.93(9); 2007 Sep https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1955040/
4. Ermoshkin VI. New theory of arrhythmia. Conceptual substantiation of arrhythmia mechanisms. Cardiometry; Issue 8; May 2016; p.6–17; doi:10.12710/cardiometry.2016.8.617.
5. Ermoshkin VI. A New Theory of Certain Cardiovascular Diseases. EC Cardiology, Volume 2 Issue 5 November 2016 https://www.ecronicon.com/eccy/pdf/ECCY-02-000034.pdf
6. Ermoshkin V (2016) Pathological Role of the "Invisible" Anastomoses. J Bioengineer & Biomedical Sci 6: 209. doi: 10.4172/2155-9538.1000209 http://www.omicsonline.org/open-access/pathological-role-of-the-invisible-anastomoses-2155-9538-1000209.pdf
7. Ermoshkin VI. Heart transplantation mysteriously eliminates arrhythmia. Cardiometry; Issue 8; May 2016; p.18–21; doi:10.12710/cardiometry.2016.8.1821.
8. Ermoshkin VI. The mechanism of bronchial asthma. Why do the most serious asthma attacks occur at night? EC Cardiology, Volume 2 Issue 4 November 2016 https://www.ecronicon.com/eccy/pdf/ECCY-02-000030.pdf
9. Ermoshkin VI. Proceedings of the International Conference on arrhythmia. [Abstract] Australia. Brisbane. Arrhythmia-2016, July 14-15, 2016, http://arrhythmias.conferenceseries.com/scientific-program/ (Day 1, 16:45-17: 00).
Rhodaline Yayra Odoi
V.N. Karazin National University, Ukraine
Title: Obesity and the heart: Obesity management
Time : 11:45-12:15

Biography:
Rhodaline Yayra Odoi is a second-year Medical student at V.N. Karazin National University, Ukraine where she is currently pursuing General Medicine. She is looking forward to specialize in Pediatrics/Gynecology and Obstetrics after medical school. She has previously volunteered at Tema General Hospital (Tema-Ghana), Korle-Bu Teaching Hospital (Accra-Ghana), where she worked at the Pediatrics and Gynecology and Obstetrics department. She is looking forward to work with Hamad General hospital (Doha, Qatar).
Abstract:
Statement of the Problem: Obesity which is excess fatty weight is one of the most predominant cardiovascular risk factor. The association between obesity and cardiovascular disease is complex and not limited to the standard risk factors like hypertension, dyslipidemia, and type 2 diabetes mellitus. Recent years researches have shown that obesity causes most cardiovascular diseases through mechanisms like subclinical inflammation, endothelial dysfunction, increased sympathetic tone, atherogenic lipid profiles, enhanced thrombogenic factors, and also through obstructive sleep apnea. The purpose of this study is to create awareness of obesity, its bad outcome and to awaken us on the lifestyle we live.
Methodological & Theoretical Orientation: Based on my research done in Ghana, people with issues of obesity have cardiovascular disease.
Findings: Several studies have shown association between obesity and prognosis among those with coronary disease and heart failure, this may be due to limitations of ways we define obesity. There are numerous data suggesting that measuring central obesity or total body fat content might be more appropriate than using the body mass index method alone.
Conclusion: The management of obesity is challenging and studies using lifestyle modification alone or with pharmacologic agents generally have limited success and high levels of weight regain. Bariatric surgery has proven to be an effective and safe way to induce and maintain significant weight loss but its limited to those with medically complicated obesity or people who are severely obese therefore urging imminent doctors and medical practitioners to thoroughly educate their patients who are obese or yet to get obese on the bad effect of obesity and also encourage them to exercise regularly which may reduce their weight and also help boost their metabolic activities hence keeping them healthy.
Image:
Figure 1: Pathophysiology of obesity and cardiovascular disease.
References:
1. acobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300-2.
2. Catenacci VA, Hill JO, Wyatt H.R. The obesity epidemic. Clin Chest Med. 2009;30:415-44.
3. OECD Press Release 2010: Health: OECD says government must fight fat. Available at: http://www.oecd.org/document/35/0,3343,en_21571361 _44315115_46064099_1_1_1_1,00.html.
4. Poirier P. Cardiologists and abdominal obesity: lost in translation? Heart. 2009;95:1033-5.
5. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881-7.
Umme Habiba Ferdaushi
National Institute of Cardiovascular Diseases (NICVD), Bangladesh
Title: Short term outcome of cardiac resynchronization therapy on functional recovery of patients with congestive heart failure in Bangladeshi population
Time : 12:15-12:45

Biography:
Umme Habiba Ferdaushi has completed her MBBS at Shere Bangla Medical College, Barisal, Bangladesh and fellowship in Cardiology at Bangladesh College of Physicians and Surgeons (BCPS), Bangladesh. She works at National Institute of Cardiovascular Diseases (NICVD), a tertiary level cardiovascular institute. She has published three papers in reputed journals.
Abstract:
Background & Aim: Cardiac resynchronization therapy (CRT) reduces symptoms and improves left ventricular function in patients with heart failure due to left ventricular systolic dysfunction and cardiac dyssynchrony. We analyzed the clinical and echocardiographic outcome of CRT in heart failure patients.
Methods: A total 35 heart failure patients were included in this prospective observational study, conducted from Feb 2015–Feb 2016 in the cardiology department of NICVD, Dhaka. Demographic profile, clinical data and investigations including coronary angiogram were done. Patients underwent CRT-P (BiV pacemaker) or CRT-D (defibrillator) and were followed up. Clinical, electrocardiographic and echocardiographic study were performed before and three months after CRT implantation.
Results: After three months of BiV pacing, New York Heart Association functional class improved from 3.3±0.44 to 1.7±0.6 (p<0.001). Left ventricular end diastolic diameter was reduced from 67.9±5.2 to 61.9±5.9 mm (p<0.001) and left ventricular end systolic diameter was reduced from 56.4±6.5 to 50.5±7.2 mm (p<0.001). Ejection fraction was significantly increased after three months from 27.5±4.3% to 38.8±6.7%, (p<0.001).The average grade of mitral regurgitation was decreased from 1.49±0.65 to 0.43±0.61 (p<0.001). The number of hospitalization was also significantly reduced from 2.51±1.44 to 0.11±0.32 (p<0.001). Among the study patients, 71.4% patient was responders, 17.1% super responders and 11.4% non-responders.
Conclusions: Although the study was performed on a small number of patients, it can be considered that CRT had favorable hemodynamic and clinical results and reduced the need for hospitalization in our heart failure patients.
Vladimir Ermoshkin
Russian New University, Russia
Title: Problems heart failure. Unexpected outcome
Time : 12:45-13:15

Biography:
Vladimir Ermoshkin completed his Graduation in Physics department at Moscow State University in 1978. He has worked at Russian New University (RosNOU) as Physicist. He has published 10 articles on Cardiology in prominent magazines (Russian and English).
Abstract:
Goal.
C делана попытка нового анализа м еханизм аAn attempt to study heart failure.
Methods.
An examination of the literature, participation in conferences, and discussions with Russian leading cardiologists.
Results.
Heart failure (HF) can be chronic (CHF) or acute (AHF). This terminology was in the 12th Congress of Physicians in 1935, more than 80 years ago. Up until now, the disease has been considered incurable and with an unknown cause, or with unknown etiology.
With current clinical position, CHF is a complex disorder with specific symptoms (dyspnea, fatigue, decreased physical activity, edema, heart rhythm and palpitations al.), which are related to inadequate perfusion of organs and tissues at rest or during exercise. Another characteristic feature of chronic heart failure is fluid retention in the body. Most often, edema begins in the lower extremities, pelvic organs and gradually rise above the body up to the heart and lungs.
Currently, the primary mechanism HF is considered the deterioration of the heart's ability to fill or empty the chambers due to damage to the myocardium, including heart attacks while with concomitant imbalance neurohormonal systems. As a rule, HF ejection fraction is reduced to 30-45%, with the required fraction of 50-65%.
The treatment of heart failure is ineffective. There is a high death rate from heart failure after diagnosis. Symptomatic heart failure occurs in 1.8-2.0% of people. Among people older than 65 years, the frequency of occurrence of heart failure is 6-10%. Further, after heart failure, the five-year survival of patients with heart failure less than 50% and the risk of sudden death in CHF is 5 times higher than in the general population.
Over the past few decades thousands of articles have been written listing the following causes: myocardial infarction, cardiac ischemia, atherosclerosis, high blood pressure (BP), disease of the heart valves, inflammatory and non-inflammatory disease of the myocardium, congenital heart defects, lung disease, alcohol abuse, drug reception, addiction to tobacco.
Thus, HF is widespread, its mechanism is unknown, the treatment of symptomatic, effective treatment of heart failure has not been found, and there is a high mortality rate.
The main conclusion of the interim etiology HF: heart failure can be a major manifestation of virtually all heart disease [1].
Heart failure necessarily occurs following a heart attack, but there are instances of heart failure where no heart attack occurred.
The new theory of cardiovascular disease is based on the fact that the arterial and venous pools may periodically be linked large anastomoses (natural shunts) [2,3,4,5,6]. In normal conditions, arteriovenous anastomoses (AVA) are closed, but during periods of increased physical and psychological stress due to increased blood pressure, they can open. The most common pathological role extended AVA manifests in the liver area. This leads to a reset of the arterial blood directly into veins. Because of this, some groups of working cells are left without sufficient food. Under certain conditions, the AVA may be open too long. As a result, blood high pressure penetrates into the venous bed and gradually fills it. Usually first affecting the liver and portal vein.
Further, high blood pressure occurs not only in the right atrium area, but throughout the vena cava, and extending downwards to the small veins. Much depends on the diameter and network location of the veins, the location of the AVA (one AVA or cascade), lifestyle, and the prevailing human posture.
Of course, a substantial pressure increase in small venules and veins occurs over a long period. In the first stage, venous valves counteract the pathological process, but after some time the venous valves begin to break down. This is due to the necessary counteraction of the total pressure of 60-70 mm Hg and above. The total pressure consists of the diastolic blood pressure and the hydrostatic pressure of the liquid column located above the valve. Due to the lack of pressure difference between arterioles and venules capillary circulation slows in some organs, or even stops. Lymph also stagnates.
In my opinion, obstacles in the form of a blood clot, tumor emboli do not raise the pressure in the veins near the venules, but add additional pressure transmitted through the veins of the confluence zone of large anastomoses [6]. Over time, because of the permeability of vascular veins occur swelling of tissues, varicose veins, venous thrombosis. Cardiology has made a mistake by confusing the cause and effect. If proper prevention is not provided, the process of the disease is enhanced, and further diseases in the small pelvis, lower limbs, and others, including some types of cancer will occur.
Yes, it is, of cancer. There are studies which have shown that the average time of the first heart attack to cancer is equal to only 2.8 years [7]. Another study shows 70% increase in the risk of cancer in patients for three years after a diagnosis of heart failure [8]. Thus, the incidence of heart failure and cancer rigidly connected and this connection is probably due to the stagnation of blood in the veins due to open AVA!
Thus, according to the new theory, the majority of cardiovascular diseases occur due to malfunction of the AVA, due to venous plethora. Extras: because pulse waves running through a crowded vena cava, with mechanical and electrical excitation arrhythmic CMC [2, 9].
Thus, in my opinion, the following provisions must be corrected in cardiology.
1. "The inadequate perfusion of the organs and tissues at rest or under load" in heart failure - is the result of open-AVA, which naturally leads to stasis capillary blood flow in some organs, perfusion to the slowdown.
2. Increased venous pressure in the vena cava and some veins of smaller caliber primarily because of the open ABA. Diseases of the heart valves and the venous valves are usually secondary.
3. "Apnea" occurs due to swelling of the lung tissue. Lungs not only have vessels of the pulmonary circulation, but also arteries and veins of the systemic circulation. At night, in a horizontal position, the excess venous pressure of a great circle reaches the lungs, because all human organs in the supine position are almost equal footing in relation to the Earth's gravity [3].
4. Reducing or maintaining ventricular ejection fraction of the heart, especially at the initial stage of development of heart failure, often does nothing. The problem of hypertension leads to dilation of the heart cavities. The statements regarding the impact of ejection fractions confirmed by the data published in the British Heart Foundation online magazine [10]. «Some people with heart failure have a normal ejection fraction, so ejection fraction is used alongside other tests to help diagnose heart failure». Additionally, there is information in the American Heart Association online journal [11]: «A significant proportion of patients with heart failure happen to have a normal ventricular ejection fraction at ECG during examination».
5. The occurrence and rate of development of heart failure is also influenced by additional human factors, including lifestyle, education level, physical activity, sedentary work, proper diet, special exercises, and genetics.
6. Apparently, there is found the explanation high values pairwise correlations between most of cardiovascular disease. Cardiovascular diseases are correlated with the some types of cancer. The reason is the "wrong" working AVA, it is in the position "open" too long and, unfortunately, are deep inside the human body.
Conclusions.
In my opinion, it is necessary to correct the errors in the field of cardiology. These errors have affected the efficiency of Cardiology for 50-100 years. It is necessary to check and discuss new proposals enabling a new era in cardiology.
References:
1. Ðаучный центр Ñердечно-ÑоÑудиÑтой хирургии им. Ð.Ð. Бакулева. Интернет реÑурÑ. 2017. http://www.bakulev.ru/cyclo/detail.php?ID=39219
2. Ermoshkin VI. New theory of arrhythmia. Conceptual substantiation of arrhythmia mechanisms. Cardiometry; Issue 8; May 2016; p.6–17; doi:10.12710/cardiometry.2016.8.617. http://www.cardiometry.net/issues/no8-may-2016/new-theory-of-arrhythmia
3. Ermoshkin VI. The mechanism of bronchial asthma. Why do the most serious asthma attacks occur at night? EC Cardiology, Volume 2 Issue 4 November 2016 https://www.ecronicon.com/eccy/pdf/ECCY-02-000030.pdf
4. Ermoshkin VI. Arteriovenous anastomoses and cardiovascular diseases. 8th Cardiovascular Nursing & Nurse Practitioners Meeting. August 08-09, 2016 Las Vegas, USA, DOI: 10.4172/2155-9880.C1.045 http://www.omicsonline.org/proceedings/arteriovenous-anastomoses-and-cardiovascular-diseases-48866.html
5. Ermoshkin VI. A New Theory of Certain Cardiovascular Diseases. EC Cardiology, Volume 2 Issue 5 November 2016 https://www.ecronicon.com/eccy/pdf/ECCY-02-000034.pdf
6. Ermoshkin VI. Venous congestion due to large arteriovenous anastomoses. 566 Chiswick High Road, London,
Greater London, W4 5YA, United Kingdom, DOI: 10.15761/HCCT.1000101 https://oatext.com/Venous-congestion-due-to-large-arteriovenous-anastomoses.php
7. Jyoty Malhotra, Paolo Boffetta. Association of increased cancer risk with heart failure. Journal of American College of Cardiology. Vol. 68, No 3, 2016.
8. Clark RA , Berry NM , Chowdhury MH , McCarthy AL , Ullah S. , Versace VL , Atherton JJ , Koczwara B , & Roder, D. (2016) Ð¡ÐµÑ€Ð´ÐµÑ‡Ð½Ð°Ñ Ð½ÐµÐ´Ð¾ÑтаточноÑÑ‚ÑŒ поÑле Ð»ÐµÑ‡ÐµÐ½Ð¸Ñ Ñ€Ð°ÐºÐ°: ХарактериÑтики, выживаемоÑти и ÑмертноÑти анализа данных , ÑвÑзанного Ñо здоровьем. Roder D. (2016) Heart failure following cancer treatment: Characteristics, survival and mortality of a linked health data analysis. Внутренние болезни журнал, 46 (11), ÑÑ‚Ñ€. 1297-1306. Internal Medicine Journal , 46 (11), pp. 1297-1306. http://eprints.qut.edu.au/101771/
9. Kamkin AG, Kiseleva IS, Yarygin VN. Fibrillation, defibrillation. Priroda. 2002;4:1040. [Russian]
10. British Heart Foundation. Heart failure. Internet resource. 2017. https://www.bhf.org.uk/heart-health/conditions/heart-failure
